当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Motif-Directed Oxidative Folding to Design and Discover Multicyclic Peptides for Protein Recognition
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2025-03-13 , DOI: 10.1021/acs.accounts.5c00060
Chuanliu Wu 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2025-03-13 , DOI: 10.1021/acs.accounts.5c00060
Chuanliu Wu 1
Affiliation
|
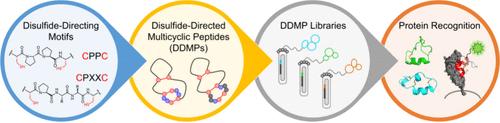
Multicyclic peptides that are constrained through covalent cross-linkers can usually maintain stable three-dimensional (3D) structures without the necessity of incorporating noncovalently interacting cores. This configuration allows for a greater utilization of residues for functional purposes compared to larger proteins, rendering multicyclic peptides attractive molecular modalities for the development of chemical tools and therapeutic agents. Even smaller multicyclic peptides, which may lack stable 3D structures due to limited sequence-driven folding capabilities, can still benefit from the specific conformations stabilized by covalent cross-linkers to facilitate target binding. Disulfide-rich peptides (DRPs) are a class of particularly significant multicyclic peptides that are primarily composed of disulfide bonds in their interior. However, the structural diversity of DRPs is limited to a few naturally occurring and designer scaffolds, which significantly impedes the development of multicyclic peptide ligands and therapeutics. To address this issue, we developed a novel method that utilizes disulfide-directing motifs to design and discover DRPs with new structures and functions in random sequence space. Compared with traditional DRPs, these new DRPs that incorporate disulfide-directing motifs exhibit more precise oxidative folding regarding disulfide pairing and demonstrate greater tolerance to sequence manipulations. Thus, we designated these peptides as disulfide-directed multicyclic peptides (DDMPs).
中文翻译:

基序导向的氧化折叠,用于设计和发现用于蛋白质识别的多环肽
通过共价交联剂限制的多环肽通常可以保持稳定的三维 (3D) 结构,而无需掺入非共价相互作用的核心。与较大的蛋白质相比,这种配置允许将残基更多地用于功能目的,使多环肽成为开发化学工具和治疗剂的有吸引力的分子模式。即使是更小的多环肽,由于序列驱动的折叠能力有限,可能缺乏稳定的 3D 结构,仍然可以受益于共价交联剂稳定的特定构象,以促进靶标结合。富二硫键肽 (DRP) 是一类特别重要的多环肽,其内部主要由二硫键组成。然而,DRP 的结构多样性仅限于少数天然存在的和设计支架,这极大地阻碍了多环肽配体和治疗剂的开发。为了解决这个问题,我们开发了一种新方法,利用二硫键导向基序在随机序列空间中设计和发现具有新结构和功能的 DRP。与传统的 DRP 相比,这些包含二硫键导向基序的新型 DRP 在二硫键配对方面表现出更精确的氧化折叠,并且对序列作表现出更大的耐受性。因此,我们将这些肽命名为二硫键定向多环肽 (DDMPs)。
更新日期:2025-03-14
中文翻译:

基序导向的氧化折叠,用于设计和发现用于蛋白质识别的多环肽
通过共价交联剂限制的多环肽通常可以保持稳定的三维 (3D) 结构,而无需掺入非共价相互作用的核心。与较大的蛋白质相比,这种配置允许将残基更多地用于功能目的,使多环肽成为开发化学工具和治疗剂的有吸引力的分子模式。即使是更小的多环肽,由于序列驱动的折叠能力有限,可能缺乏稳定的 3D 结构,仍然可以受益于共价交联剂稳定的特定构象,以促进靶标结合。富二硫键肽 (DRP) 是一类特别重要的多环肽,其内部主要由二硫键组成。然而,DRP 的结构多样性仅限于少数天然存在的和设计支架,这极大地阻碍了多环肽配体和治疗剂的开发。为了解决这个问题,我们开发了一种新方法,利用二硫键导向基序在随机序列空间中设计和发现具有新结构和功能的 DRP。与传统的 DRP 相比,这些包含二硫键导向基序的新型 DRP 在二硫键配对方面表现出更精确的氧化折叠,并且对序列作表现出更大的耐受性。因此,我们将这些肽命名为二硫键定向多环肽 (DDMPs)。







































 京公网安备 11010802027423号
京公网安备 11010802027423号