当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into the Challenges of Cycling a Nonaqueous Na–O2 Battery
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2016-11-14 00:00:00 , DOI: 10.1021/acs.jpclett.6b02267 Tao Liu 1 , Gunwoo Kim 1, 2 , Mike T. L. Casford 1 , Clare P. Grey 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2016-11-14 00:00:00 , DOI: 10.1021/acs.jpclett.6b02267 Tao Liu 1 , Gunwoo Kim 1, 2 , Mike T. L. Casford 1 , Clare P. Grey 1
Affiliation
|
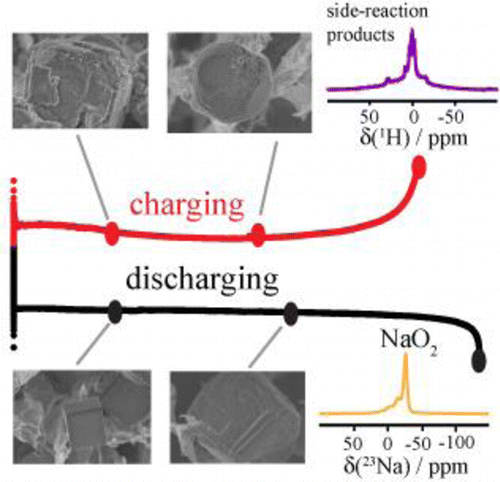
Superoxide-based nonaqueous metal–oxygen batteries have received considerable research attention as they exhibit high energy densities and round-trip efficiencies. The cycling performance, however, is still poor. Here we study the cycling characteristic of a Na–O2 battery using solid-state nuclear magnetic resonance, Raman spectroscopy, and scanning electron microscopy. We find that the poor cycling performance is primarily caused by the considerable side reactions stemming from the chemical aggressiveness of NaO2 as both a solid-phase and dissolved species in the electrolyte. The side reaction products cover electrode surfaces and hinder electron transfer across the electrode–electrolyte interface, being a major reason for cell failure. In addition, the available electrode surface and porosity change considerably during cell discharging and charging, affecting the diffusion of soluble species (superoxide and water) and resulting in inhomogeneous reactions across the electrode. This study provides insights into the challenges associated with achieving long-lived superoxide-based metal–O2 batteries.
中文翻译:

循环非水Na–O 2电池挑战的机械原理
基于超氧化物的非水金属氧气电池由于具有高能量密度和往返效率而受到了相当多的研究关注。但是,循环性能仍然很差。在这里,我们使用固态核磁共振,拉曼光谱和扫描电子显微镜研究了Na–O 2电池的循环特性。我们发现不良的循环性能主要是由NaO 2的化学侵蚀性引起的大量副反应引起的既是固相又是溶解在电解质中的物质。副反应产物覆盖电极表面,并阻碍电子跨电极-电解质界面的转移,这是电池失效的主要原因。此外,在电池放电和充电期间,可用的电极表面和孔隙率会发生很大变化,从而影响可溶性物质(超氧化物和水)的扩散,并导致整个电极的反应不均匀。这项研究提供了与获得长寿命超氧化物基金属O 2电池相关的挑战的见解。
更新日期:2016-11-14
中文翻译:

循环非水Na–O 2电池挑战的机械原理
基于超氧化物的非水金属氧气电池由于具有高能量密度和往返效率而受到了相当多的研究关注。但是,循环性能仍然很差。在这里,我们使用固态核磁共振,拉曼光谱和扫描电子显微镜研究了Na–O 2电池的循环特性。我们发现不良的循环性能主要是由NaO 2的化学侵蚀性引起的大量副反应引起的既是固相又是溶解在电解质中的物质。副反应产物覆盖电极表面,并阻碍电子跨电极-电解质界面的转移,这是电池失效的主要原因。此外,在电池放电和充电期间,可用的电极表面和孔隙率会发生很大变化,从而影响可溶性物质(超氧化物和水)的扩散,并导致整个电极的反应不均匀。这项研究提供了与获得长寿命超氧化物基金属O 2电池相关的挑战的见解。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号