当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Paired Electrolysis in the Simultaneous Production of Synthetic Intermediates and Substrates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-11-14 , DOI: 10.1021/jacs.6b08667 Mark J. Llorente 1 , Bichlien H. Nguyen 2 , Clifford P. Kubiak 1 , Kevin D. Moeller 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-11-14 , DOI: 10.1021/jacs.6b08667 Mark J. Llorente 1 , Bichlien H. Nguyen 2 , Clifford P. Kubiak 1 , Kevin D. Moeller 2
Affiliation
|
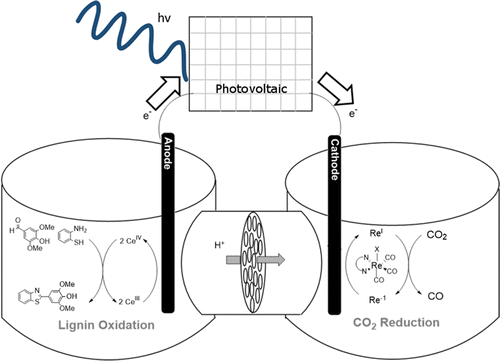
In electrochemical processes, an oxidation half-reaction is always paired with a reduction half-reaction. Although systems for reactions such as the reduction of CO2 can be coupled to water oxidation to produce O2 at the anode, large-scale O2 production is of limited value. One may replace a low-value half-reaction with a compatible half-reaction that can produce a valuable chemical compound and operate at a lower potential. In doing so, both the anodic and cathodic half-reactions yield desirable products with a decreased energy demand. Here we demonstrate a paired electrolysis in the case of the oxidative condensation of syringaldehyde and o-phenylenediamine to give 2-(3,5-dimethoxy-4-hydroxyphenyl)benzimidazole coupled with the reduction of CO2 to CO mediated by molecular electrocatalysts. We also present general principles for evaluating current-voltage characteristics and power demands in paired electrolyzers.
中文翻译:

同时生产合成中间体和底物的配对电解
在电化学过程中,氧化半反应总是与还原半反应配对。尽管诸如还原 CO2 等反应系统可以与水氧化耦合以在阳极产生 O2,但大规模 O2 生产的价值有限。人们可以用兼容的半反应代替低价值的半反应,后者可以产生有价值的化合物并在较低的电位下运行。这样做时,阳极和阴极半反应都会产生所需的产品,同时降低了能量需求。在这里,我们展示了在丁香醛和邻苯二胺氧化缩合得到 2-(3,5-二甲氧基-4-羟基苯基)苯并咪唑的情况下的配对电解,同时通过分子电催化剂将 CO2 还原为 CO。
更新日期:2016-11-14
中文翻译:

同时生产合成中间体和底物的配对电解
在电化学过程中,氧化半反应总是与还原半反应配对。尽管诸如还原 CO2 等反应系统可以与水氧化耦合以在阳极产生 O2,但大规模 O2 生产的价值有限。人们可以用兼容的半反应代替低价值的半反应,后者可以产生有价值的化合物并在较低的电位下运行。这样做时,阳极和阴极半反应都会产生所需的产品,同时降低了能量需求。在这里,我们展示了在丁香醛和邻苯二胺氧化缩合得到 2-(3,5-二甲氧基-4-羟基苯基)苯并咪唑的情况下的配对电解,同时通过分子电催化剂将 CO2 还原为 CO。































 京公网安备 11010802027423号
京公网安备 11010802027423号