当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation and optimization of metal- and organocatalyzed decarboxylative radical addition of carboxylic acids to Seebach-Beckwith dehydroalanine towards optically pure unnatural amino acids
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2025-02-20 , DOI: 10.1016/j.tetlet.2025.155507
Ilia Perov , Karizza F. Catenza , Jose Jr. Abucay , Yu-Ting Hsiao , John C. Vederas
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2025-02-20 , DOI: 10.1016/j.tetlet.2025.155507
Ilia Perov , Karizza F. Catenza , Jose Jr. Abucay , Yu-Ting Hsiao , John C. Vederas
|
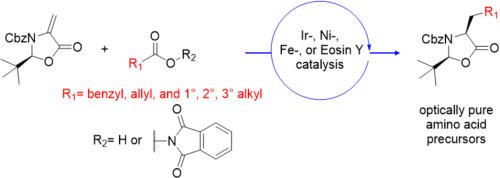
Four decarboxylative conjugate radical addition methods, including recent and greener methods using Fe- and Eosin Y as photocatalysts, were examined using Seebach-Beckwith dehydroalanine as a radical acceptor. Head-to-head comparison of yields and purity suggests that Ir-photocatalyzed decarboxylation of carboxylic acids is the optimal method for syntheses involving resonance-stabilized radicals. However, approaches relying on cheaper Ni-, Fe-, and Eosin Y-catalyzed processes are quite viable for the conjugate addition of 1°, 2°, and 3° radicals.
中文翻译:

金属和有机催化脱羧自由基将羧酸加成到 Seebach-Beckwith 脱氢丙氨酸中对光学纯非天然氨基酸的研究和优化
使用脱氢丙氨酸作为自由基受体的 Seebach-Beck 研究了四种脱羧偶联自由基加成方法,包括使用 Fe- 和伊红 Y 作为光催化剂的最新和更绿色的方法。产率和纯度的头对头比较表明,Ir 光催化羧酸脱羧是涉及共振稳定自由基合成的最佳方法。然而,依赖于更便宜的 Ni-、Fe-和曙红 Y 催化过程的方法对于偶联添加 1°、2° 和 3° 自由基是相当可行的。
更新日期:2025-02-20
中文翻译:

金属和有机催化脱羧自由基将羧酸加成到 Seebach-Beckwith 脱氢丙氨酸中对光学纯非天然氨基酸的研究和优化
使用脱氢丙氨酸作为自由基受体的 Seebach-Beck 研究了四种脱羧偶联自由基加成方法,包括使用 Fe- 和伊红 Y 作为光催化剂的最新和更绿色的方法。产率和纯度的头对头比较表明,Ir 光催化羧酸脱羧是涉及共振稳定自由基合成的最佳方法。然而,依赖于更便宜的 Ni-、Fe-和曙红 Y 催化过程的方法对于偶联添加 1°、2° 和 3° 自由基是相当可行的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号