当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deoxyfluorination of alcohols with 3,3-difluoro-1,2-diarylcyclopropenes.
Nature Communications ( IF 14.7 ) Pub Date : 2016-11-14 , DOI: 10.1038/ncomms13320 Lingchun Li , Chuanfa Ni , Fei Wang , Jinbo Hu
Nature Communications ( IF 14.7 ) Pub Date : 2016-11-14 , DOI: 10.1038/ncomms13320 Lingchun Li , Chuanfa Ni , Fei Wang , Jinbo Hu
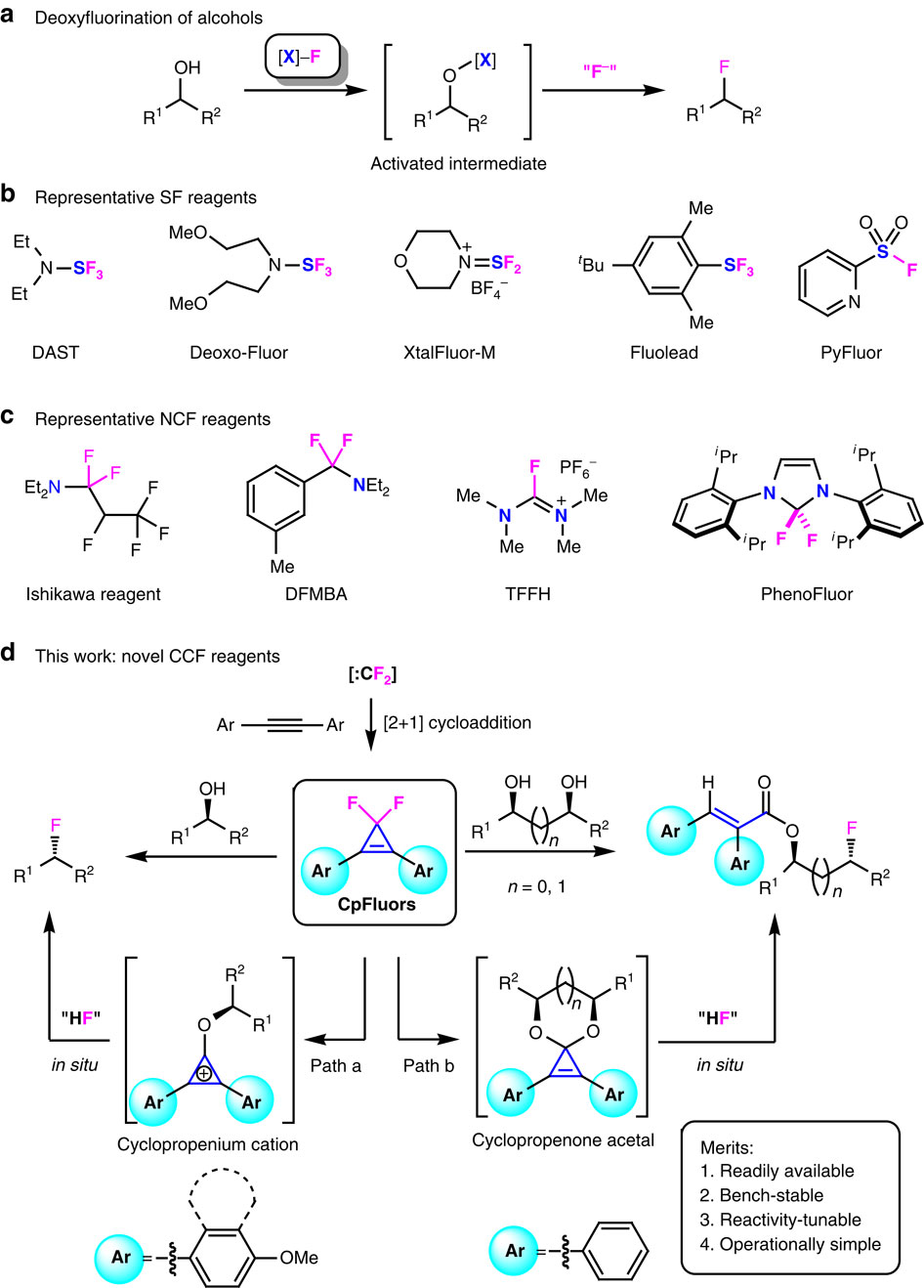
|
Aromatic cation activation is a useful strategy to promote deoxyfunctionalization; however, the deoxyfluorination of alcohols with cyclopropenium cation remains an unsolved problem due to the weak nucleophilicity of fluoride ion. Here we report the use of 3,3-difluoro-1,2-diarylcyclopropenes (CpFluors) as easily accessible and reactivity-tunable deoxyfluorination reagents. The electronic nature of CpFluors is critical for fluorination of monoalcohols via alkoxycyclopropenium cations, and CpFluors with electron-rich aryl substituents facilitate the transformation with high efficiency; however, selective monofluorination of 1,2- and 1,3-diols, which proceeds via cyclopropenone acetals, is less dependent on the electronic nature of CpFluors. Moreover, CpFluors are more sensitive to the electronic nature of alcohols than many other deoxyfluorination reagents, thus fluorination of longer diols can be achieved selectively at the relatively electron-rich position. This research not only unveils the first example of deoxyfluorination reagents that contain an all-carbon scaffold, but also sheds light on the divergent reactivity of cyclopropenium cation in deoxyfunctionalization of alcohols.
中文翻译:

用3,3-二氟-1,2-二芳基环丙烯对醇进行脱氧氟化。
芳族阳离子活化是促进脱氧官能化的有用策略。然而,由于氟离子的弱亲核性,醇与环丙烯阳离子的脱氧氟化作用仍未解决。在这里,我们报告了3,3-二氟-1,2-二芳基环丙烯(CpFluors)的使用,它们是易于获得且可调节反应性的脱氧氟化试剂。CpFluors的电子性质对于通过烷氧基环丙烯阳离子氟化一元醇至关重要,带有富电子芳基取代基的CpFluors可以高效地促进转化;但是,通过环丙烯酮缩醛进行的1,2-和1,3-二醇的选择性单氟化反应,对CpFluors的电子性质的依赖性较小。而且,与许多其他脱氧氟化试剂相比,CpFluor对醇的电子性质更敏感,因此可以在相对富电子的位置选择性地实现更长二醇的氟化。这项研究不仅揭示了包含全碳支架的脱氧氟化试剂的第一个实例,而且揭示了环丙阳离子在醇的脱氧官能化中的发散反应性。
更新日期:2016-11-16
中文翻译:

用3,3-二氟-1,2-二芳基环丙烯对醇进行脱氧氟化。
芳族阳离子活化是促进脱氧官能化的有用策略。然而,由于氟离子的弱亲核性,醇与环丙烯阳离子的脱氧氟化作用仍未解决。在这里,我们报告了3,3-二氟-1,2-二芳基环丙烯(CpFluors)的使用,它们是易于获得且可调节反应性的脱氧氟化试剂。CpFluors的电子性质对于通过烷氧基环丙烯阳离子氟化一元醇至关重要,带有富电子芳基取代基的CpFluors可以高效地促进转化;但是,通过环丙烯酮缩醛进行的1,2-和1,3-二醇的选择性单氟化反应,对CpFluors的电子性质的依赖性较小。而且,与许多其他脱氧氟化试剂相比,CpFluor对醇的电子性质更敏感,因此可以在相对富电子的位置选择性地实现更长二醇的氟化。这项研究不仅揭示了包含全碳支架的脱氧氟化试剂的第一个实例,而且揭示了环丙阳离子在醇的脱氧官能化中的发散反应性。































 京公网安备 11010802027423号
京公网安备 11010802027423号