Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionizable Lipids with Optimized Linkers Enable Lung-Specific, Lipid Nanoparticle-Mediated mRNA Delivery for Treatment of Metastatic Lung Tumors
ACS Nano ( IF 15.8 ) Pub Date : 2025-02-06 , DOI: 10.1021/acsnano.4c18636
Gonna Somu Naidu, Riccardo Rampado, Preeti Sharma, Assaf Ezra, Govinda Reddy Kundoor, Dor Breier, Dan Peer
ACS Nano ( IF 15.8 ) Pub Date : 2025-02-06 , DOI: 10.1021/acsnano.4c18636
Gonna Somu Naidu, Riccardo Rampado, Preeti Sharma, Assaf Ezra, Govinda Reddy Kundoor, Dor Breier, Dan Peer
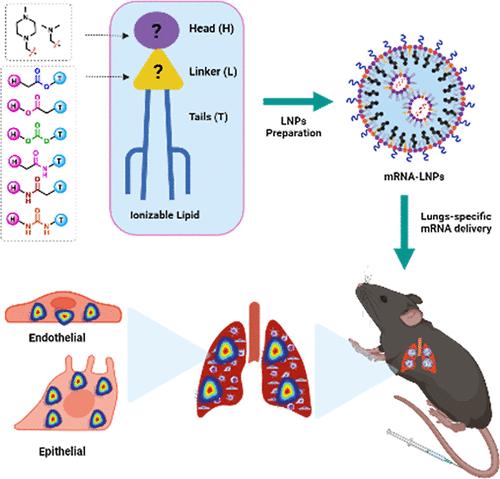
|
Lipid nanoparticles (LNPs) have emerged as a groundbreaking delivery system for vaccines and therapeutic mRNAs. Ionizable lipids are the most pivotal component of LNPs due to their ability to electrostatically interact with mRNA, allowing its encapsulation while concurrently enabling its endosomal escape following cellular internalization. Thus, extensive research has been performed to optimize the ionizable lipid structure and to develop formulations that are well tolerated and allow efficient targeting of different organs that result in a high and sustained mRNA expression. However, one facet of the ionizable lipids’ structure has been mostly overlooked: the linker segment between the ionizable headgroup and their tails. Here, we screened a rationally designed library of ionizable lipids with different biodegradable linkers. We extensively characterized LNPs formulated using these ionizable lipids and elucidated how these minor structural changes in the ionizable lipids structure radically influenced the LNPs’ biodistribution in vivo. We showed how the use of amide and urea linkers can modulate the LNPs’ pKa, resulting in an improved specificity for lung transfection. Finally, we demonstrated how one of these lipids (lipid 35) that form LNPs entrapping a bacterial toxin [pseudomonas exotoxin A (mmPE)] in the form of an mRNA reduced tumor burden and significantly increased the survival of mice with lung metastasis.
中文翻译:

具有优化接头的可电离脂质可实现肺特异性脂质纳米颗粒介导的 mRNA 递送,用于治疗转移性肺肿瘤
脂质纳米颗粒 (LNP) 已成为疫苗和治疗性 mRNA 的开创性递送系统。可电离脂质是 LNP 最关键的成分,因为它们能够与 mRNA 静电相互作用,允许其封装,同时在细胞内化后使其内体逃逸。因此,已经进行了广泛的研究,以优化可电离的脂质结构,并开发耐受性良好的配方,并允许有效靶向不同的器官,从而产生高且持续的 mRNA 表达。然而,可电离脂质结构的一个方面大多被忽视了:可电离头部基团与其尾部之间的接头段。在这里,我们筛选了一个合理设计的具有不同可生物降解接头的可电离脂质库。我们广泛表征了使用这些可电离脂质配制的 LNP,并阐明了可电离脂质结构中的这些微小结构变化如何从根本上影响 LNP 在体内的生物分布。我们展示了酰胺和尿素接头的使用如何调节 LNP 的 pKa,从而提高肺转染的特异性。最后,我们展示了这些脂质之一 (脂质 35) 如何形成 LNP,以 mRNA 的形式捕获细菌毒素 [假单胞菌外毒素 A (mmPE)] ] 如何降低肿瘤负荷并显着提高肺转移小鼠的存活率。
更新日期:2025-02-06
中文翻译:

具有优化接头的可电离脂质可实现肺特异性脂质纳米颗粒介导的 mRNA 递送,用于治疗转移性肺肿瘤
脂质纳米颗粒 (LNP) 已成为疫苗和治疗性 mRNA 的开创性递送系统。可电离脂质是 LNP 最关键的成分,因为它们能够与 mRNA 静电相互作用,允许其封装,同时在细胞内化后使其内体逃逸。因此,已经进行了广泛的研究,以优化可电离的脂质结构,并开发耐受性良好的配方,并允许有效靶向不同的器官,从而产生高且持续的 mRNA 表达。然而,可电离脂质结构的一个方面大多被忽视了:可电离头部基团与其尾部之间的接头段。在这里,我们筛选了一个合理设计的具有不同可生物降解接头的可电离脂质库。我们广泛表征了使用这些可电离脂质配制的 LNP,并阐明了可电离脂质结构中的这些微小结构变化如何从根本上影响 LNP 在体内的生物分布。我们展示了酰胺和尿素接头的使用如何调节 LNP 的 pKa,从而提高肺转染的特异性。最后,我们展示了这些脂质之一 (脂质 35) 如何形成 LNP,以 mRNA 的形式捕获细菌毒素 [假单胞菌外毒素 A (mmPE)] ] 如何降低肿瘤负荷并显着提高肺转移小鼠的存活率。

































 京公网安备 11010802027423号
京公网安备 11010802027423号