当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spatial Control of CAR T Cell Activation Using Tumor-Homing Polymers
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-02-04 , DOI: 10.1021/jacs.4c15442
Clinton M. Heinze, Trey J. Pichon, Abe Y. Wu, Michael Baldwin, James Matthaei, Kefan Song, Meilyn Sylvestre, Joshua Gustafson, Nathan J. White, Michael C. Jensen, Suzie H. Pun
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-02-04 , DOI: 10.1021/jacs.4c15442
Clinton M. Heinze, Trey J. Pichon, Abe Y. Wu, Michael Baldwin, James Matthaei, Kefan Song, Meilyn Sylvestre, Joshua Gustafson, Nathan J. White, Michael C. Jensen, Suzie H. Pun
|
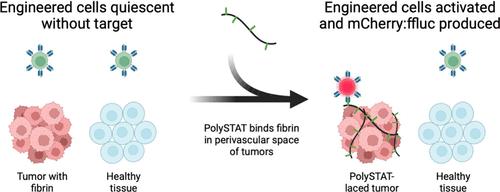
CAR T cell therapies often lack specificity, leading to issues ranging from inadequate antigen targeting to off-tumor toxicities. To counter that lack of specificity, we expanded tumor targeting capabilities with universal CAR and spatially defined CAR T cell engagement with targets through a combination of synthetic biology and biomaterial approaches. We developed a novel framework, called “In situ Mobilization: Polymer Activated Cell Therapies” (IMPACT) for polymer-mediated, anatomical control of IF-THEN gated CAR T cells. With IMPACT, a regulated payload such as a BiTE or tumor-targeting CAR will only be expressed after engineered cells engage a tumor-localizing polymer (“IF” condition). In this first demonstration of IMPACT, we engineered CAR T cells to respond to fluorescein that is displayed by an injectable polymer that binds to and is retained in fibrin deposits in tumor microenvironments. This interaction then drives selective and conditional expression of a protein within tumors (“THEN” condition). Here, we develop the polymer and CAR T cell infrastructure of IMPACT and demonstrate tumor-localized CAR T cell activation in a murine tumor model after the intravenous administration of polymer and engineered T cells.
中文翻译:

使用肿瘤归巢聚合物对 CAR T 细胞活化进行空间控制
CAR T 细胞疗法通常缺乏特异性,导致从抗原靶向不足到非肿瘤毒性等问题。为了解决这种特异性的缺乏,我们通过合成生物学和生物材料方法的结合,通过通用 CAR 和空间定义的 CAR T 细胞与靶标的结合来扩展肿瘤靶向能力。我们开发了一种称为“原位动员:聚合物活化细胞疗法”(IMPACT) 的新框架,用于聚合物介导的 IF-THEN 门控 CAR T 细胞的解剖学控制。使用 IMPACT,只有在工程细胞与肿瘤定位聚合物结合(“IF”条件)后,才会表达 BiTE 或肿瘤靶向 CAR 等受调节的有效载荷。在 IMPACT 的首次演示中,我们设计了 CAR T 细胞以响应荧光素,荧光素由可注射聚合物显示,该聚合物与肿瘤微环境中的纤维蛋白沉积物结合并保留在纤维蛋白沉积物中。然后,这种相互作用驱动蛋白质在肿瘤内的选择性和条件性表达(“THEN”条件)。在这里,我们开发了 IMPACT 的聚合物和 CAR T 细胞基础设施,并证明了静脉注射聚合物和工程 T 细胞后小鼠肿瘤模型中肿瘤定位的 CAR T 细胞活化。
更新日期:2025-02-04
中文翻译:

使用肿瘤归巢聚合物对 CAR T 细胞活化进行空间控制
CAR T 细胞疗法通常缺乏特异性,导致从抗原靶向不足到非肿瘤毒性等问题。为了解决这种特异性的缺乏,我们通过合成生物学和生物材料方法的结合,通过通用 CAR 和空间定义的 CAR T 细胞与靶标的结合来扩展肿瘤靶向能力。我们开发了一种称为“原位动员:聚合物活化细胞疗法”(IMPACT) 的新框架,用于聚合物介导的 IF-THEN 门控 CAR T 细胞的解剖学控制。使用 IMPACT,只有在工程细胞与肿瘤定位聚合物结合(“IF”条件)后,才会表达 BiTE 或肿瘤靶向 CAR 等受调节的有效载荷。在 IMPACT 的首次演示中,我们设计了 CAR T 细胞以响应荧光素,荧光素由可注射聚合物显示,该聚合物与肿瘤微环境中的纤维蛋白沉积物结合并保留在纤维蛋白沉积物中。然后,这种相互作用驱动蛋白质在肿瘤内的选择性和条件性表达(“THEN”条件)。在这里,我们开发了 IMPACT 的聚合物和 CAR T 细胞基础设施,并证明了静脉注射聚合物和工程 T 细胞后小鼠肿瘤模型中肿瘤定位的 CAR T 细胞活化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号