当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of a Nonheme Iron Cyclopropanase with a Homologous Hydroxylase Reveals Mechanistic Features Associated with Distinct Reaction Outcomes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-02-03 , DOI: 10.1021/jacs.4c17741
Yu-Cong Zheng, Xiaojun Li, Lide Cha, Jared C. Paris, Charalambos Michael, Richiro Ushimaru, Yasushi Ogasawara, Ikuro Abe, Yisong Guo, Wei-chen Chang
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-02-03 , DOI: 10.1021/jacs.4c17741
Yu-Cong Zheng, Xiaojun Li, Lide Cha, Jared C. Paris, Charalambos Michael, Richiro Ushimaru, Yasushi Ogasawara, Ikuro Abe, Yisong Guo, Wei-chen Chang
|
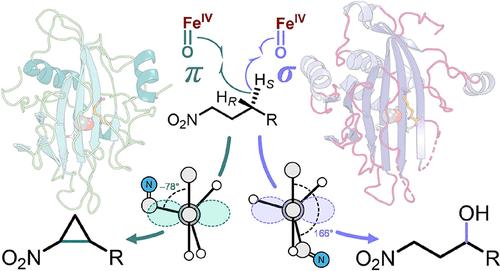
Despite the diversity of reactions catalyzed by mononuclear iron and 2-oxoglutarate-dependent enzymes, the factors that lead to diverse reaction outcomes beyond canonical hydroxylation remain elusive. Cyclopropanation reactions are of particular interest not only due to the prevalence of cyclopropane moieties in pharmaceuticals but also due to the chemistry that allows cyclopropanation to outcompete oxygen rebound. HrmJ is one such cyclopropanase from the biosynthetic pathway of hormaomycin; however, a homologue is herein discovered that instead catalyzes C-hydroxylation of the same nitro enolate substrate. These enzymes were reconstituted with Mn(II) and V(IV)═O as mimics of the resting (Fe(II)) and reactive (Fe(IV)═O) intermediate states, respectively. Corresponding crystal structures of the cyclopropanase bound with a substrate imply H atom transfer via an offline π-pathway. In contrast, analogous structural analysis of the hydroxylase implies H atom abstraction likely proceeds through a σ-pathway. Preparation of isotopically labeled substrates and stopped-flow kinetic analyses indicate that while the pro-S hydrogen of C4 is abstracted in both enzymes, the Fe(IV)═O intermediate reacts ca. 17-fold faster in the active site of the hydroxylase, consistent with the mechanistic assignments. These results also support a correlation between the mechanism of H atom transfer and the subsequent fate of the substrate radical once generated. A subtle difference in substrate positioning not only affects the H atom abstraction pathway but also allows the nitro enolate moiety to intercept the resulting substrate radical in the active site of the cyclopropase, thereby facilitating intramolecular C–C bond formation in a stereoselective manner.
中文翻译:

非血红素铁环丙烷酶与同源羟化酶的比较揭示了与不同反应结果相关的机制特征
尽管单核铁和 2-氧代戊二酸依赖性酶催化的反应多种多样,但导致经典羟基化之外的不同反应结果的因素仍然难以捉摸。环丙烷化反应特别令人感兴趣,不仅因为环丙烷部分在药物中普遍存在,还因为允许环丙烷化超过氧反弹的化学性质。HrmJ 是来自激素霉素生物合成途径的一种这样的环丙烷酶;然而,本文发现了一种同系物,它反而催化相同硝基烯醇酯底物的 C-羟基化。这些酶分别用 Mn(II) 和 V(IV)═O 重构,作为静息 (Fe(II)) 和反应性 (Fe(IV)═O) 中间态的模拟物。与底物结合的环丙烷酶的相应晶体结构意味着 H 原子通过离线 π 途径转移。相比之下,羟化酶的类似结构分析表明 H 原子提取可能通过 σ 途径进行。同位素标记底物的制备和停流动力学分析表明,虽然 C4 的 pro-S 氢在两种酶中都被提取出来,但 Fe(IV)═O 中间体在羟化酶的活性位点的反应速度快约 17 倍,这与机制分配一致。这些结果还支持 H 原子转移机制与底物自由基产生后的后续命运之间的相关性。底物定位的细微差异不仅影响 H 原子提取途径,还允许硝基烯醇化物部分在环螺旋桨酶的活性位点拦截产生的底物自由基,从而以立体选择性方式促进分子内 C-C 键的形成。
更新日期:2025-02-03
中文翻译:

非血红素铁环丙烷酶与同源羟化酶的比较揭示了与不同反应结果相关的机制特征
尽管单核铁和 2-氧代戊二酸依赖性酶催化的反应多种多样,但导致经典羟基化之外的不同反应结果的因素仍然难以捉摸。环丙烷化反应特别令人感兴趣,不仅因为环丙烷部分在药物中普遍存在,还因为允许环丙烷化超过氧反弹的化学性质。HrmJ 是来自激素霉素生物合成途径的一种这样的环丙烷酶;然而,本文发现了一种同系物,它反而催化相同硝基烯醇酯底物的 C-羟基化。这些酶分别用 Mn(II) 和 V(IV)═O 重构,作为静息 (Fe(II)) 和反应性 (Fe(IV)═O) 中间态的模拟物。与底物结合的环丙烷酶的相应晶体结构意味着 H 原子通过离线 π 途径转移。相比之下,羟化酶的类似结构分析表明 H 原子提取可能通过 σ 途径进行。同位素标记底物的制备和停流动力学分析表明,虽然 C4 的 pro-S 氢在两种酶中都被提取出来,但 Fe(IV)═O 中间体在羟化酶的活性位点的反应速度快约 17 倍,这与机制分配一致。这些结果还支持 H 原子转移机制与底物自由基产生后的后续命运之间的相关性。底物定位的细微差异不仅影响 H 原子提取途径,还允许硝基烯醇化物部分在环螺旋桨酶的活性位点拦截产生的底物自由基,从而以立体选择性方式促进分子内 C-C 键的形成。

































 京公网安备 11010802027423号
京公网安备 11010802027423号