当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Insights into the Selectivity of Single-Atom Fe–N–C Catalysts for Electrochemical NOx Reduction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-02-02 , DOI: 10.1021/jacs.4c14021
Yao Tan, Junwei Fu, Tao Luo, Kang Liu, Min Liu
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-02-02 , DOI: 10.1021/jacs.4c14021
Yao Tan, Junwei Fu, Tao Luo, Kang Liu, Min Liu
|
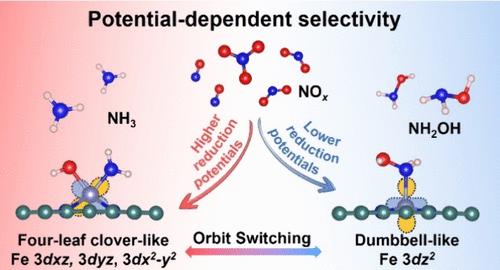
Single-atom Fe–N–C catalysts have attracted significant attention in the NOx reduction reaction (NOxRR). However, the origin of their selectivity in the NOxRR remains unclear, impeding further advancements in application. Herein, we investigate the potential-driven competitive mechanism for NH3 and NH2OH production in the NOxRR over single-atom pyridinic-FeN4 and pyrrolic-FeN4 sites using constant-potential density functional theory calculations. The origin of selectivity in the NOxRR is linked to the switching of Fe 3d orbitals as they interact with intermediates. The selectivity between NH3 and NH2OH is determined by the applied potentials. The pyridinic-FeN4 predominantly generates NH3 at higher reduction potentials (−0.6 to −1.2 V, vs SHE), while NH2OH is favored at lower reduction potentials (0.6 to −0.6 V). The pyrrolic-FeN4 shows a similar potential-dependent product distribution, with a crossover potential of −1.0 V. The selectivity-determining intermediates (SDIs) in the NOxRR are *NH2OH and *NH2 + *OH. The potential-dependent selectivity is governed by the switching of Fe 3d orbitals interacting with SDIs, from dumbbell-shaped Fe 3dz2 to four-leaf clover-like Fe 3dxz, 3dyz, and 3dx2-y2, which plays a crucial role in controlling product distribution based on applied potentials. These findings offer new insights into the product selectivity of single-atom catalysts for the NOxRR.
中文翻译:

单原子 Fe–N–C 催化剂电化学 NOx 还原选择性的理论见解
单原子 Fe-N-C 催化剂在 NOx 还原反应 (NOxRR) 中引起了极大的关注。然而,它们在 NOxRR 中的选择性来源仍不清楚,阻碍了应用的进一步进展。在此,我们使用恒电位密度泛函理论计算研究了单原子吡啶-FeN4 和吡咯-FeN4 位点上 NOxRR 中产生 NH3 和 NH2OH 的电位驱动竞争机制。NOxRR 中选择性的来源与 Fe 3d 轨道在与中间体相互作用时的切换有关。NH3 和 NH2OH 之间的选择性由施加的电位决定。吡啶-FeN4 主要在较高的还原电位(-0.6 至 -1.2 V,与 SHE 相比)下产生 NH3,而 NH2OH 在较低的还原电位(0.6 至 -0.6 V)下更受欢迎。吡咯酸 FeN4 显示出类似的电位依赖性产物分布,交越电位为 −1.0 V。NOxRR 中的选择性决定中间体 (SDI) 为 *NH2OH 和 *NH2 + *OH。电位依赖性选择性由与 SDI 相互作用的 Fe 3d 轨道的切换控制,从哑铃形 Fe 3dz2 到四叶草状 Fe 3dxz、3dyz 和 3dx2-y 2,这在控制基于施加电位的产物分布中起着至关重要的作用。 这些发现为单原子催化剂对 NOxRR 的产物选择性提供了新的见解。
更新日期:2025-02-02
中文翻译:

单原子 Fe–N–C 催化剂电化学 NOx 还原选择性的理论见解
单原子 Fe-N-C 催化剂在 NOx 还原反应 (NOxRR) 中引起了极大的关注。然而,它们在 NOxRR 中的选择性来源仍不清楚,阻碍了应用的进一步进展。在此,我们使用恒电位密度泛函理论计算研究了单原子吡啶-FeN4 和吡咯-FeN4 位点上 NOxRR 中产生 NH3 和 NH2OH 的电位驱动竞争机制。NOxRR 中选择性的来源与 Fe 3d 轨道在与中间体相互作用时的切换有关。NH3 和 NH2OH 之间的选择性由施加的电位决定。吡啶-FeN4 主要在较高的还原电位(-0.6 至 -1.2 V,与 SHE 相比)下产生 NH3,而 NH2OH 在较低的还原电位(0.6 至 -0.6 V)下更受欢迎。吡咯酸 FeN4 显示出类似的电位依赖性产物分布,交越电位为 −1.0 V。NOxRR 中的选择性决定中间体 (SDI) 为 *NH2OH 和 *NH2 + *OH。电位依赖性选择性由与 SDI 相互作用的 Fe 3d 轨道的切换控制,从哑铃形 Fe 3dz2 到四叶草状 Fe 3dxz、3dyz 和 3dx2-y 2,这在控制基于施加电位的产物分布中起着至关重要的作用。 这些发现为单原子催化剂对 NOxRR 的产物选择性提供了新的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号