当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic electrode reconfiguration promotes in situ electrochemical peracetic acid synthesis for selective water decontamination
Water Research ( IF 11.4 ) Pub Date : 2025-01-28 , DOI: 10.1016/j.watres.2025.123205
Hanlin Yan, Xiaoguang Liu, Yang Zong, Zhendong Lei, Qunbiao He, Zhenyu Zhao, Zhengwei Zhou, Guojie Ye, Chengsi Hou, Deli Wu
Water Research ( IF 11.4 ) Pub Date : 2025-01-28 , DOI: 10.1016/j.watres.2025.123205
Hanlin Yan, Xiaoguang Liu, Yang Zong, Zhendong Lei, Qunbiao He, Zhenyu Zhao, Zhengwei Zhou, Guojie Ye, Chengsi Hou, Deli Wu
|
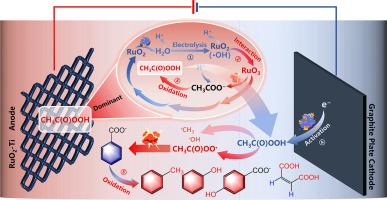
In situ synthesis and activation of peracetic acid (PAA) for water decontamination is a promising way to overcome the transport and storage problems in PAA applications. Here, an in situ electrochemical PAA synthesis and activation system is constructed using RuO2−Ti “active” electrode and graphite plate as the anode and the cathode, respectively. PAA is efficiently generated at the RuO2−Ti anode with a maximum real-time concentration of ∼1020 μM and a negligible precursor loss of 2.91 % after 180 min, and can be activated at the cathode to destruct a refractory pollutant (i.e., benzoic acid (BA)) with the rate constant of 0.22−0.28 h−1, even under the interference of co-existing anions. Multiple pieces of evidence, including differential electrochemical mass spectrometry, sulfoxide probing test, and electron paramagnetic resonance spectroscopy, indicate that the oxygen-atom-transferring oxidation of CH3COO− by a high-valent ruthenium-oxo intermediate (i.e., RuO3) in situ formed through the electrode reconfiguration between RuO2 and chem-sorbed HO• mainly accounts for PAA synthesis. Acetylperoxyl radical (CH3C(O)OO•) was evidenced as the dominant species for BA degradation. This study proposes an in situ strategy to electrochemically synthesize and activate PAA for selective water decontamination and enriches the understandings of the mechanism of “active” electrode in peroxide synthesis.
中文翻译:

动态电极重配置促进原位电化学过氧乙酸合成,用于选择性水净化
用于水净化的过氧乙酸 (PAA) 的原位合成和活化是克服 PAA 应用中运输和储存问题的一种很有前途的方法。在这里,使用 RuO 2 −Ti “活性”电极和石墨板分别作为阳极和阴极构建了原位电化学 PAA 合成和活化系统。PAA 在 RuO 2 − Ti 阳极高效生成,最大实时浓度为 ∼1020 μM,180 分钟后前驱体损失为 2.91% 可忽略不计,并且可以在阴极激活以破坏难降解污染物(即苯甲酸 (BA)),速率常数为 0.22−0.28 h −1 ,即使在共存阴离子的干扰下也是如此。包括差分电化学质谱、亚砜探测试验和电子顺磁共振光谱在内的多项证据表明,通过 RuO 2 和化学吸附 HO • 之间的电极重新构型原位形成的高价钌-氧代中间体(即 RuO 3 )对 CH 3 COO − 的氧原子转移氧化是 PAA 合成的主要原因。乙酰过氧自由基 (CH 3 C(O)OO • ) 被证明是 BA 降解的优势物种。本研究提出了一种电化学合成和激活 PAA 以进行选择性水净化的原位策略,并丰富了对过氧化物合成中“活性”电极机制的理解。
更新日期:2025-02-01
中文翻译:

动态电极重配置促进原位电化学过氧乙酸合成,用于选择性水净化
用于水净化的过氧乙酸 (PAA) 的原位合成和活化是克服 PAA 应用中运输和储存问题的一种很有前途的方法。在这里,使用 RuO 2 −Ti “活性”电极和石墨板分别作为阳极和阴极构建了原位电化学 PAA 合成和活化系统。PAA 在 RuO 2 − Ti 阳极高效生成,最大实时浓度为 ∼1020 μM,180 分钟后前驱体损失为 2.91% 可忽略不计,并且可以在阴极激活以破坏难降解污染物(即苯甲酸 (BA)),速率常数为 0.22−0.28 h −1 ,即使在共存阴离子的干扰下也是如此。包括差分电化学质谱、亚砜探测试验和电子顺磁共振光谱在内的多项证据表明,通过 RuO 2 和化学吸附 HO • 之间的电极重新构型原位形成的高价钌-氧代中间体(即 RuO 3 )对 CH 3 COO − 的氧原子转移氧化是 PAA 合成的主要原因。乙酰过氧自由基 (CH 3 C(O)OO • ) 被证明是 BA 降解的优势物种。本研究提出了一种电化学合成和激活 PAA 以进行选择性水净化的原位策略,并丰富了对过氧化物合成中“活性”电极机制的理解。































 京公网安备 11010802027423号
京公网安备 11010802027423号