当前位置:
X-MOL 学术
›
Coord. Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural effects of the Pb2+ 6s2 lone pair activity: Eccentricity
Coordination Chemistry Reviews ( IF 20.3 ) Pub Date : 2025-01-22 , DOI: 10.1016/j.ccr.2025.216434
Charlene Harriswangler, Antía Freire-García, Saray Argibay-Otero, Aurora Rodríguez-Rodríguez, José M. Rodríguez, David Esteban-Gómez, Ezequiel M. Vázquez-López, Carlos Platas-Iglesias
Coordination Chemistry Reviews ( IF 20.3 ) Pub Date : 2025-01-22 , DOI: 10.1016/j.ccr.2025.216434
Charlene Harriswangler, Antía Freire-García, Saray Argibay-Otero, Aurora Rodríguez-Rodríguez, José M. Rodríguez, David Esteban-Gómez, Ezequiel M. Vázquez-López, Carlos Platas-Iglesias
|
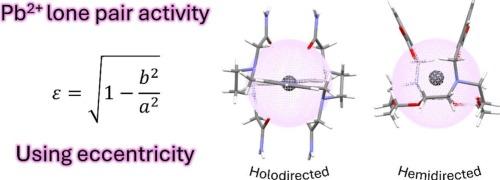
The stereochemical activity of the 6s2 lone pair has profound consequences on the coordination chemistry of metal ions like Tl+ , Pb2+ and Bi3+ . The coordination chemistry of Pb2+ is very likely the most extensively investigated among the metal ions with [Xe]4f14 5d10 6s2 electronic configuration, allowing for a detailed analysis of the factors that affect the stereochemical activity of the 6s2 lone pair. In this review, the X-ray structures of a wide variety of Pb2+ complexes were analyzed by approximating their coordination environments to a prolate spheroid. The eccentricity (ε) of the spheroid provides a straightforward measure of the stereochemical activity of the Pb2+ lone pair. Holodirected structures are characterized by a rather spherical distribution of the donor atoms around the metal ion, leading to values of ε < 0.05. The stereochemical activity of the lone pair may result in either moderately hemidirected structures, with ε values in the range 0.05–0.15, or strongly hemidirected structures, when ε > 0.15. Hemidirected structures are favored by low coordination numbers and the presence of negatively charged donor atoms. In this work not only have many representative complexes been analyzed to provide a classification of these systems according to their eccentricity, but also a simple tool is provided for coordination chemists, through which they can easily classify their complexes as holodirected or hemidirected.
中文翻译:

Pb2+ 6s2 孤对电子活性的结构效应:偏心率
6s2 孤对电子的立体化学活性对 Tl+、Pb2+ 和 Bi3+ 等金属离子的配位化学有深远的影响。Pb2+ 的配位化学很可能是具有 [Xe]4f145d106s2 电子构型的金属离子中研究最广泛的,从而可以详细分析影响 6s2 孤对电子立体化学活性的因素。在这篇综述中,通过将各种 Pb2+ 复合物的配位环境近似为长球来分析它们的 X 射线结构。球体的偏心率 (ε) 提供了 Pb2+ 孤对电子立体化学活性的直接测量。全向结构的特征是供体原子围绕金属离子呈相当球形分布,导致值ε < 0.05。孤对电子的立体化学活性可能导致中等半向结构,ε值在 0.05-0.15 范围内,或当 > 为 0.15 时ε强半向结构。半向结构受到低配位数和带负电荷的供体原子的存在的影响。在这项工作中,不仅分析了许多具有代表性的复合物,以根据它们的偏心率对这些系统进行分类,而且还为配位化学家提供了一个简单的工具,通过该工具,他们可以轻松地将复合物分类为全向或半向。
更新日期:2025-01-22
中文翻译:

Pb2+ 6s2 孤对电子活性的结构效应:偏心率
6s2 孤对电子的立体化学活性对 Tl+、Pb2+ 和 Bi3+ 等金属离子的配位化学有深远的影响。Pb2+ 的配位化学很可能是具有 [Xe]4f145d106s2 电子构型的金属离子中研究最广泛的,从而可以详细分析影响 6s2 孤对电子立体化学活性的因素。在这篇综述中,通过将各种 Pb2+ 复合物的配位环境近似为长球来分析它们的 X 射线结构。球体的偏心率 (ε) 提供了 Pb2+ 孤对电子立体化学活性的直接测量。全向结构的特征是供体原子围绕金属离子呈相当球形分布,导致值ε < 0.05。孤对电子的立体化学活性可能导致中等半向结构,ε值在 0.05-0.15 范围内,或当 > 为 0.15 时ε强半向结构。半向结构受到低配位数和带负电荷的供体原子的存在的影响。在这项工作中,不仅分析了许多具有代表性的复合物,以根据它们的偏心率对这些系统进行分类,而且还为配位化学家提供了一个简单的工具,通过该工具,他们可以轻松地将复合物分类为全向或半向。































 京公网安备 11010802027423号
京公网安备 11010802027423号