当前位置:
X-MOL 学术
›
Pest Manag. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and development of pyrazol‐5‐ylbenzamide derivatives containing chiral oxazoline moiety as fungicides based on molecular docking
Pest Management Science ( IF 3.8 ) Pub Date : 2025-01-16 , DOI: 10.1002/ps.8663
Xiang Cheng, Zhen Zhang, Yuanjian Huang, Fanglei Wang, Dandan Wang, Xianhai Lv, Xihao Chang
Pest Management Science ( IF 3.8 ) Pub Date : 2025-01-16 , DOI: 10.1002/ps.8663
Xiang Cheng, Zhen Zhang, Yuanjian Huang, Fanglei Wang, Dandan Wang, Xianhai Lv, Xihao Chang
|
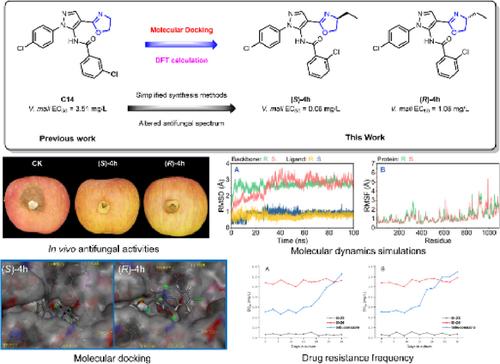
BACKGROUNDDevelopment of novel chiral antifungal agents for effective control of plant pathogens is urgently needed. In this study, a series of pyrazol‐5‐yl‐benzamide derivatives containing chiral oxazoline moiety were rationally designed and developed based on molecular docking.RESULTSThe in vitro antifungal assay results indicated that compounds (rac )‐4h (R1 = Et), (S )‐4 h (R1 = S ‐Et) and (R )‐4 h (R1 = R ‐Et) exhibited remarkable antifungal activities against Valsa mali with median effective concentration (EC50 ) values of 0.24, 0.06 and 1.08 mg/L, respectively. Preliminary structure–activity relationships (SARs) revealed that the modification of the chiral substituent group at the oxazoline moiety significantly affected the antifungal activities of the target compounds. Furthermore, compounds (S )‐4h (87.5%) and (R )‐4h (84.3%) exhibited in vivo protective activities comparable to tebuconazole (87.5%) against V. mali . Subsequent molecular docking analysis, succinate dehydrogenase (SDH) enzyme inhibition assays and molecular dynamic (MD) simulations verified that the potential target enzyme of this class of derivatives could be SDH and helped to explain the large difference in antifungal activities of compounds (S )‐4h and (R )‐4h. Confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM) observations confirmed that these two compounds severely disrupted the mycelial morphology of V. mali . Theoretical calculation studies provided some insight into the subsequent modification of such pyrazol‐5‐yl‐benzamide derivatives. Resistance frequency studies showed that (S )‐4h and (R )‐4h treatments were less likely to produce resistant fungal strains than tebuconazole. Meanwhile, compounds (S )‐4h and (R )‐4h exhibited no apparent toxicity to the Apis mellifera L. population.CONCLUSIONTherefore, these derivatives are potential candidates for the development of novel chiral fungicides for crop protection. © 2025 Society of Chemical Industry.
中文翻译:

基于分子对接的含手性噁唑啉部分作为杀菌剂的吡唑-5-基苯甲酰胺衍生物的设计与开发
背景迫切需要开发新型手性抗真菌剂以有效控制植物病原体。本研究基于分子对接合理设计开发了一系列含有手性噁唑啉部分的吡唑-5-基-苯甲酰胺衍生物。结果体外抗真菌试验结果表明,化合物 (rac)‐4h (R1 = Et)、(S)‐4 h (R1 = S‐Et) 和 (R)‐4 h (R1 = R‐Et) 对苹果缬草表现出显著的抗真菌活性,中位有效浓度 (EC50) 值分别为 0.24、0.06 和 1.08 mg/L。初步构效关系 (SARs) 显示,恶唑啉部分手性取代基的修饰显着影响目标化合物的抗真菌活性。此外,化合物 (S)‐4h (87.5%) 和 (R)‐4h (84.3%) 在体内表现出与戊唑醇 (87.5%) 相当的对马里弧菌的保护活性。随后的分子对接分析、琥珀酸脱氢酶 (SDH) 酶抑制测定和分子动力学 (MD) 模拟验证了这类衍生物的潜在靶酶可能是 SDH,并有助于解释化合物 (S)-4h 和 (R)-4h 的抗真菌活性的巨大差异。共聚焦激光扫描显微镜 (CLSM) 和扫描电子显微镜 (SEM) 观察证实,这两种化合物严重破坏了 V. mali 的菌丝形态。理论计算研究为此类吡唑-5-基-苯甲酰胺衍生物的后续修饰提供了一些见解。耐药频率研究表明,与戊唑醇相比,(S)‐4h 和 (R)‐4h 处理不太可能产生耐药性真菌菌株。同时,化合物 (S)‐4h 和 (R)‐4h 对 Apis mellifera L 无明显毒性。 人口。结论因此,这些衍生物是开发用于作物保护的新型手性杀菌剂的潜在候选者。© 2025 化工学会.
更新日期:2025-01-16
中文翻译:

基于分子对接的含手性噁唑啉部分作为杀菌剂的吡唑-5-基苯甲酰胺衍生物的设计与开发
背景迫切需要开发新型手性抗真菌剂以有效控制植物病原体。本研究基于分子对接合理设计开发了一系列含有手性噁唑啉部分的吡唑-5-基-苯甲酰胺衍生物。结果体外抗真菌试验结果表明,化合物 (rac)‐4h (R1 = Et)、(S)‐4 h (R1 = S‐Et) 和 (R)‐4 h (R1 = R‐Et) 对苹果缬草表现出显著的抗真菌活性,中位有效浓度 (EC50) 值分别为 0.24、0.06 和 1.08 mg/L。初步构效关系 (SARs) 显示,恶唑啉部分手性取代基的修饰显着影响目标化合物的抗真菌活性。此外,化合物 (S)‐4h (87.5%) 和 (R)‐4h (84.3%) 在体内表现出与戊唑醇 (87.5%) 相当的对马里弧菌的保护活性。随后的分子对接分析、琥珀酸脱氢酶 (SDH) 酶抑制测定和分子动力学 (MD) 模拟验证了这类衍生物的潜在靶酶可能是 SDH,并有助于解释化合物 (S)-4h 和 (R)-4h 的抗真菌活性的巨大差异。共聚焦激光扫描显微镜 (CLSM) 和扫描电子显微镜 (SEM) 观察证实,这两种化合物严重破坏了 V. mali 的菌丝形态。理论计算研究为此类吡唑-5-基-苯甲酰胺衍生物的后续修饰提供了一些见解。耐药频率研究表明,与戊唑醇相比,(S)‐4h 和 (R)‐4h 处理不太可能产生耐药性真菌菌株。同时,化合物 (S)‐4h 和 (R)‐4h 对 Apis mellifera L 无明显毒性。 人口。结论因此,这些衍生物是开发用于作物保护的新型手性杀菌剂的潜在候选者。© 2025 化工学会.































 京公网安备 11010802027423号
京公网安备 11010802027423号