当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Enantioselective Three-Component Fluoroalkylalkynylation of Unactivated Alkenes
ACS Catalysis ( IF 11.3 ) Pub Date : 2025-01-16 , DOI: 10.1021/acscatal.4c06641
Mengxia Liao 1 , Cuihuan Geng 2, 3 , Zhengze Wu 1 , Chunxiang Pan 1 , Chenwei Wang 1 , Guanghui Meng 1 , Xiaoyan Zuo 1 , Ying Zhu 1 , Xiaotian Qi 3 , Guozhu Zhang 1 , Rui Guo 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2025-01-16 , DOI: 10.1021/acscatal.4c06641
Mengxia Liao 1 , Cuihuan Geng 2, 3 , Zhengze Wu 1 , Chunxiang Pan 1 , Chenwei Wang 1 , Guanghui Meng 1 , Xiaoyan Zuo 1 , Ying Zhu 1 , Xiaotian Qi 3 , Guozhu Zhang 1 , Rui Guo 1
Affiliation
|
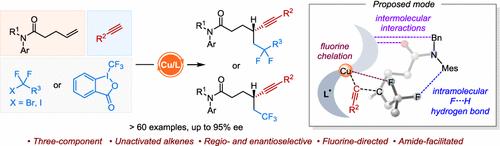
The enantioselective three-component dicarbonfunctionalization of electronically unactivated alkenes continues to pose a significant challenge. In this work, a copper-catalyzed highly regio- and enantioselective fluoroalkylalkynylation of unactivated alkenes with diverse terminal alkynes and fluoroalkyl halides under mild conditions is developed. In addition to fluoroalkyl halides, Togni’s reagent can also participate in the reaction, delivering chiral β-trifluoromethyl alkynes with high enantioselectivities. This method exhibits good functional group tolerance, facilitating the late-stage derivatization of a variety of biologically active molecules. The success of this chemistry was achieved by using a bulky indene-substituted BOPA ligand. DFT calculations indicate that the radical fluoroalkylalkynylation is achieved through a fluorine-directed outer-sphere pathway. Mechanistic studies reveal that the amide group is crucial for achieving high stereoselectivities because the exclusive F···H hydrogen bonding between the fluoroalkyl group and the Mes group on the amide can be formed to stabilize the Si-radical coupling transition state.
中文翻译:

铜催化的对映选择性三组分氟烷基炔基化反应
电子未活化烯烃的对映选择性三组分二碳官能化继续构成重大挑战。在这项工作中,开发了一种铜催化的未活化烯烃与多种末端炔烃和氟烷基卤化物的高区域和对映选择性氟烷基炔基化反应。除了氟烷基卤化物外,Togni 的试剂还可以参与反应,提供具有高对映选择性的手性 β-三氟甲基炔烃。该方法具有良好的官能团耐受性,有利于各种生物活性分子的后期衍生化。这种化学反应的成功是通过使用大体积的茚取代的 BOPA 配体实现的。DFT 计算表明,自由基氟烷基炔基化是通过氟定向的外球途径实现的。机理研究表明,酰胺基团对于实现高立体选择性至关重要,因为独有的 F···氟烷基和酰胺上的 Mes 基团之间可以形成 H 氢键,以稳定 Si 自由基偶联过渡态。
更新日期:2025-01-16
中文翻译:

铜催化的对映选择性三组分氟烷基炔基化反应
电子未活化烯烃的对映选择性三组分二碳官能化继续构成重大挑战。在这项工作中,开发了一种铜催化的未活化烯烃与多种末端炔烃和氟烷基卤化物的高区域和对映选择性氟烷基炔基化反应。除了氟烷基卤化物外,Togni 的试剂还可以参与反应,提供具有高对映选择性的手性 β-三氟甲基炔烃。该方法具有良好的官能团耐受性,有利于各种生物活性分子的后期衍生化。这种化学反应的成功是通过使用大体积的茚取代的 BOPA 配体实现的。DFT 计算表明,自由基氟烷基炔基化是通过氟定向的外球途径实现的。机理研究表明,酰胺基团对于实现高立体选择性至关重要,因为独有的 F···氟烷基和酰胺上的 Mes 基团之间可以形成 H 氢键,以稳定 Si 自由基偶联过渡态。

































 京公网安备 11010802027423号
京公网安备 11010802027423号