当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Glycerol Conversion over Bifunctional Montmorillonite-Supported Mo−V Oxide Catalysts: Insights into the Dehydration-Hydrogen Transfer Reaction Pathway Toward Allyl Alcohol
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2025-01-13 , DOI: 10.1021/acs.iecr.4c02838 Alfin Kurniawan, Jian Qiang Zhong, Ze Zhen Wang, Chun Hui Zhou
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2025-01-13 , DOI: 10.1021/acs.iecr.4c02838 Alfin Kurniawan, Jian Qiang Zhong, Ze Zhen Wang, Chun Hui Zhou
|
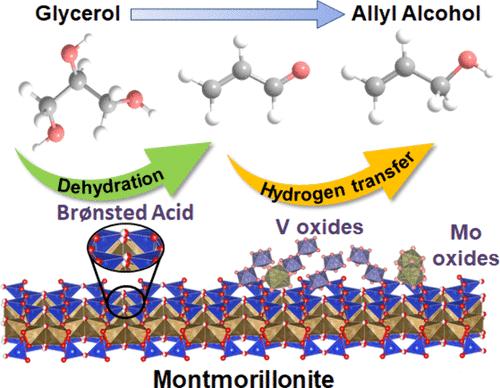
Allyl alcohol, an industrially valuable intermediate in organic synthesis, is conventionally produced through selective hydrogenation of acrolein and hydrolysis of chloropropene. However, these processes currently face challenges due to the unsustainability of fossil feedstocks. A more sustainable production of allyl alcohol from renewable feedstock (i.e., glycerol) has been demonstrated through gas-phase catalytic reaction over metal oxide catalysts with and without external H2. Improved understanding of the reaction mechanism of glycerol to allyl alcohol conversion is an important effort to rationally design more efficient catalysts. In this work, we investigate gas-phase conversion of glycerol to allyl alcohol through consecutive dehydration and hydrogen transfer reactions over Mo–V oxides supported on acid-modified montmorillonite (HMMT). The use of HMMT as a support enables the well dispersion of metal oxide particles with nominal loadings up to 5 wt % on the catalyst surface while the acidic sites facilitate dehydration of glycerol to acrolein with ∼74% selectivity. By modulating the nominal total Mo+V loading, Mo–V/HMMT catalyst with a 10 wt % metal loading afforded allyl alcohol in 22% yield at 320 °C and ambient pressure. X-ray photoelectron spectroscopic analysis of this bifunctional catalyst in the as-prepared and post-catalysis (spent) states showed changes in the surface Mo(VI)═O species and V4+/V5+ redox ratio; the latter was found to decrease due to the involvement of lower valence V4+ species in the hydrogen transfer reaction of acrolein. Meanwhile, the amount of surface Mo(VI)═O species with weak electronic metal–support interaction decreases after the reaction. Catalytic tests using an aqueous acrolein feed solution suggested the role of water as a hydride donor in transfer hydrogenation of this unsaturated aldehyde to allyl alcohol, while the addition of alcohol-containing compounds, such as 2-propanol and 1,2-propanediol leads to lower allyl alcohol selectivity with time on stream. The results presented here could guide the development of improved supported Mo−V oxide catalysts and reaction systems for efficient gas-phase glycerol conversion to allyl alcohol.
中文翻译:

双功能蒙脱石负载的 Mo-V 氧化物催化剂上的催化甘油转化:深入了解烯丙醇的脱水-氢转移反应途径
烯丙醇是一种具有工业价值的有机合成中间体,通常通过丙烯醛的选择性加氢和氯丙烯的水解来生产。然而,由于化石原料的不可持续性,这些过程目前面临挑战。通过在有和没有外部 H2 的金属氧化物催化剂上进行气相催化反应,已经证明从可再生原料(即甘油)中生产烯丙醇更可持续。更好地了解甘油向烯丙醇的反应机理是合理设计更高效催化剂的重要努力。在这项工作中,我们研究了甘油在酸改性蒙脱土 (HMMT) 负载的 Mo-V 氧化物上通过连续脱水和氢转移反应将甘油转化为烯丙醇。使用 HMMT 作为载体,可以使标称负载量高达 5 wt % 的金属氧化物颗粒在催化剂表面的良好分散,而酸性位点则有助于甘油脱水为丙烯醛,选择性约为 74%。通过调节标称总 Mo+V 负载量,金属负载量为 10 wt % 的 Mo–V/HMMT 催化剂在 320 °C 和环境压力下以 22% 的产率提供烯丙醇。这种双功能催化剂在制备状态和后催化 (spent) 状态下的 X 射线光电子能谱分析显示表面 Mo(VI)═O 种类和 V4+/V5+ 氧化还原比发生变化;由于较低价 V4+ 物质参与丙烯醛的氢转移反应,发现后者降低。同时,反应后具有弱电子金属-载体相互作用的表面 Mo(VI)═O 物质的数量减少。 使用丙烯醛水进料溶液的催化测试表明,水在将这种不饱和醛氢化转化为烯丙醇中起着氢化物供体的作用,而添加含醇的化合物(如 2-丙醇和 1,2-丙二醇)会导致烯丙醇选择性随运行时间的增加而降低。本文介绍的结果表明可以指导改进的负载型 Mo-V 氧化物催化剂和反应系统的开发,以实现高效的气相甘油转化为烯丙醇。
更新日期:2025-01-13
中文翻译:

双功能蒙脱石负载的 Mo-V 氧化物催化剂上的催化甘油转化:深入了解烯丙醇的脱水-氢转移反应途径
烯丙醇是一种具有工业价值的有机合成中间体,通常通过丙烯醛的选择性加氢和氯丙烯的水解来生产。然而,由于化石原料的不可持续性,这些过程目前面临挑战。通过在有和没有外部 H2 的金属氧化物催化剂上进行气相催化反应,已经证明从可再生原料(即甘油)中生产烯丙醇更可持续。更好地了解甘油向烯丙醇的反应机理是合理设计更高效催化剂的重要努力。在这项工作中,我们研究了甘油在酸改性蒙脱土 (HMMT) 负载的 Mo-V 氧化物上通过连续脱水和氢转移反应将甘油转化为烯丙醇。使用 HMMT 作为载体,可以使标称负载量高达 5 wt % 的金属氧化物颗粒在催化剂表面的良好分散,而酸性位点则有助于甘油脱水为丙烯醛,选择性约为 74%。通过调节标称总 Mo+V 负载量,金属负载量为 10 wt % 的 Mo–V/HMMT 催化剂在 320 °C 和环境压力下以 22% 的产率提供烯丙醇。这种双功能催化剂在制备状态和后催化 (spent) 状态下的 X 射线光电子能谱分析显示表面 Mo(VI)═O 种类和 V4+/V5+ 氧化还原比发生变化;由于较低价 V4+ 物质参与丙烯醛的氢转移反应,发现后者降低。同时,反应后具有弱电子金属-载体相互作用的表面 Mo(VI)═O 物质的数量减少。 使用丙烯醛水进料溶液的催化测试表明,水在将这种不饱和醛氢化转化为烯丙醇中起着氢化物供体的作用,而添加含醇的化合物(如 2-丙醇和 1,2-丙二醇)会导致烯丙醇选择性随运行时间的增加而降低。本文介绍的结果表明可以指导改进的负载型 Mo-V 氧化物催化剂和反应系统的开发,以实现高效的气相甘油转化为烯丙醇。































 京公网安备 11010802027423号
京公网安备 11010802027423号