当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Enantioselective Bidentate Auxiliary Directed Palladium‐Catalyzed Benzylic C−H Arylation of Amines Using a BINOL Phosphate Ligand
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2016-11-11 , DOI: 10.1002/anie.201609337
Hao Wang 1 , Hua-Rong Tong 1 , Gang He 1 , Gong Chen 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2016-11-11 , DOI: 10.1002/anie.201609337
Hao Wang 1 , Hua-Rong Tong 1 , Gang He 1 , Gong Chen 1, 2
Affiliation
|
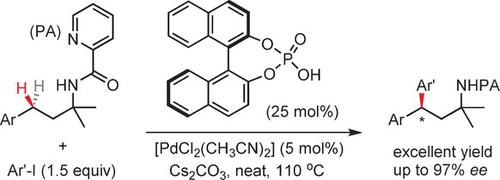
A new enantioselective palladium(II)‐catalyzed benzylic C−H arylation reaction of amines is enabled by the bidentate picolinamide (PA) directing group. This reaction provides the first example of enantioselective benzylic γ‐C−H arylations of alkyl amines, and proceeds with up to 97 % ee. The 2,2′‐dihydroxy‐1,1′‐binaphthyl (BINOL) phosphoric acid ligand, Cs2CO3, and solvent‐free conditions are essential for high enantioselectivity. Mechanistic studies suggest that multiple BINOL ligands are involved in the stereodetermining C−H palladation step.
中文翻译:

使用BINOL磷酸盐配体的胺的对映选择性二齿辅助定向钯催化苯甲酰化C-H胺化反应
二齿吡啶甲酰胺(PA)导向基团可实现新的对映选择性钯(II)催化的胺的苄基CH芳基化反应。该反应提供了烷基胺对映选择性苄基γ-C-H芳基化的第一个实例,并以高达97%的ee进行。2,2'-二羟基-1,1'-联萘(BINOL)磷酸配体Cs 2 CO 3和无溶剂条件对于高对映选择性至关重要。机理研究表明,多个BINOL配体参与立体确定CH palpalation步骤。
更新日期:2016-11-11
中文翻译:

使用BINOL磷酸盐配体的胺的对映选择性二齿辅助定向钯催化苯甲酰化C-H胺化反应
二齿吡啶甲酰胺(PA)导向基团可实现新的对映选择性钯(II)催化的胺的苄基CH芳基化反应。该反应提供了烷基胺对映选择性苄基γ-C-H芳基化的第一个实例,并以高达97%的ee进行。2,2'-二羟基-1,1'-联萘(BINOL)磷酸配体Cs 2 CO 3和无溶剂条件对于高对映选择性至关重要。机理研究表明,多个BINOL配体参与立体确定CH palpalation步骤。

































 京公网安备 11010802027423号
京公网安备 11010802027423号