当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into Liquid-Phase Titration of Palladium Surfaces
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2025-01-06 , DOI: 10.1021/acs.iecr.4c03213 Cole W. Hullfish, Rachel A. Yang, Michele L. Sarazen
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2025-01-06 , DOI: 10.1021/acs.iecr.4c03213 Cole W. Hullfish, Rachel A. Yang, Michele L. Sarazen
|
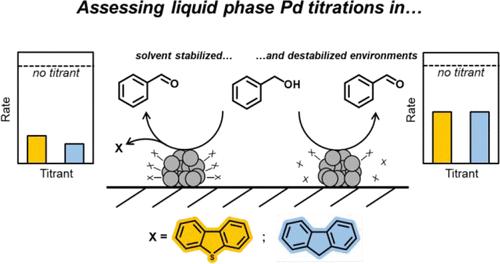
Chemisorption of strongly bound adsorbents to catalyst surfaces has been utilized extensively for site titration in the gas phase; however, extension of these molecular surface interactions to the liquid phase is not straightforward. Here, metal surface titration by solvated aromatic organothiols and hydrocarbons was studied on silica-supported Pd nanoparticle catalysts (Pd/SiO2) during batch benzyl alcohol (BA) oxidation reactions. Competitive effects of reversible titrant adsorption, titrant stabilization in varying environments, and reactant accessibility to titrated surfaces are evaluated to determine the primary drivers of benzaldehyde formation rates (rBzH) under varying titrant concentrations, solvents, and Pd oxidation states. In neat BA solvent, rBzH remains nonzero even at dibenzothiophene (DBT) and fluorene (DBT analogue without sulfur) loadings that exceed total metal atoms by a factor of 6, thus suggesting reversible titrant adsorption–desorption that is corroborated by Fourier transform infrared spectroscopy. Further depression of rBzH at titrant/Pdtot = 100:1 is consistent with titrant adsorption–desorption that is quasi-equilibrated, as well as titrant binding energies and selective site titration that influence apparent activation barriers. Effects of titrant stabilization by solvent molecules are further realized in BA oxidation reactions in n-decane, in which titrant solvation in the bulk liquid is less favored relative to BA, yielding more favorable titrant adsorption efficiency. Overall, titration efficiency in the liquid phase is found to be the net result of titrant adsorption configuration, binding energy, and stabilization by solvent molecules in the bulk that influence the relative favorability of adsorption and desorption.
中文翻译:

深入了解钯表面的液相滴定
强结合吸附剂对催化剂表面的化学吸附已广泛用于气相中的位点滴定;然而,将这些分子表面相互作用扩展到液相并不简单。在这里,在间歇苯甲醇 (BA) 氧化反应过程中,在二氧化硅负载的 Pd 纳米颗粒催化剂 (Pd/SiO2) 上研究了溶剂化芳香族有机硫醇和碳氢化合物的金属表面滴定。评估可逆滴定剂吸附、不同环境中的滴定剂稳定性以及反应物对滴定表面的可及性之间的竞争效应,以确定在不同滴定剂浓度、溶剂和 Pd 氧化态下苯甲醛生成速率 (rBzH) 的主要驱动因素。在纯 BA 溶剂中,即使在二苯并噻吩 (DBT) 和芴(不含硫的 DBT 类似物)负载量超过总金属原子 6 倍时,rBzH 仍保持非零状态,因此表明可逆滴定剂吸附-解吸,傅里叶变换红外光谱证实了这一点。在滴定剂/Pdtot = 100:1 时 rBzH 的进一步降低与准平衡的滴定剂吸附-解吸以及影响明显激活屏障的滴定剂结合能和选择性位点滴定一致。在正癸烷的 BA 氧化反应中,溶剂分子对滴定剂的稳定效果进一步实现,其中相对于 BA,本体液体中的滴定剂溶剂化不太有利,从而产生更有利的滴定剂吸附效率。 总体而言,液相中的滴定效率是滴定剂吸附构型、结合能和溶剂分子在体内稳定作用的最终结果,这些因素会影响吸附和解吸的相对有利性。
更新日期:2025-01-07
中文翻译:

深入了解钯表面的液相滴定
强结合吸附剂对催化剂表面的化学吸附已广泛用于气相中的位点滴定;然而,将这些分子表面相互作用扩展到液相并不简单。在这里,在间歇苯甲醇 (BA) 氧化反应过程中,在二氧化硅负载的 Pd 纳米颗粒催化剂 (Pd/SiO2) 上研究了溶剂化芳香族有机硫醇和碳氢化合物的金属表面滴定。评估可逆滴定剂吸附、不同环境中的滴定剂稳定性以及反应物对滴定表面的可及性之间的竞争效应,以确定在不同滴定剂浓度、溶剂和 Pd 氧化态下苯甲醛生成速率 (rBzH) 的主要驱动因素。在纯 BA 溶剂中,即使在二苯并噻吩 (DBT) 和芴(不含硫的 DBT 类似物)负载量超过总金属原子 6 倍时,rBzH 仍保持非零状态,因此表明可逆滴定剂吸附-解吸,傅里叶变换红外光谱证实了这一点。在滴定剂/Pdtot = 100:1 时 rBzH 的进一步降低与准平衡的滴定剂吸附-解吸以及影响明显激活屏障的滴定剂结合能和选择性位点滴定一致。在正癸烷的 BA 氧化反应中,溶剂分子对滴定剂的稳定效果进一步实现,其中相对于 BA,本体液体中的滴定剂溶剂化不太有利,从而产生更有利的滴定剂吸附效率。 总体而言,液相中的滴定效率是滴定剂吸附构型、结合能和溶剂分子在体内稳定作用的最终结果,这些因素会影响吸附和解吸的相对有利性。































 京公网安备 11010802027423号
京公网安备 11010802027423号