当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Limiting Halide Exchange and Doping Mn(II) in Vertex-Oriented Cube-Connected Patterned Lead Halide Perovskite Nanorods
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-12-30 , DOI: 10.1021/acs.chemmater.4c02908 Harsh Mohata, Diptam Nasipuri, Sumit Kumar Dutta, Narayan Pradhan
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-12-30 , DOI: 10.1021/acs.chemmater.4c02908 Harsh Mohata, Diptam Nasipuri, Sumit Kumar Dutta, Narayan Pradhan
|
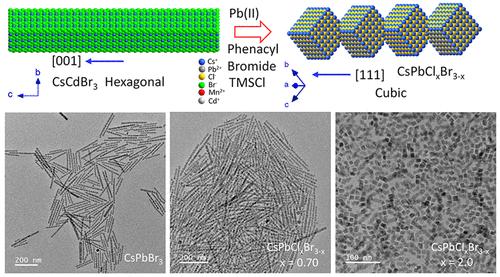
Lead halide perovskite nanocrystals remained in the forefront of inorganic optical nanomaterials for a decade. The chemistry of their formation leading to bright and phase-stable nanocrystals is also largely understood. However, this study mostly focuses on isotropic nanostructures and is limited to anisotropic shapes. Again, the study of shape anisotropy is more explored for orthorhombic CsPbBr3 and limited for the high-bandgap cubic phase CsPbCl3 nanocrystals. Keeping this in mind, herein, the halide exchange is carried out in a specific cube-connected patterned vertex-oriented nanorod of CsPbBr3 intending for complete conversion to CsPbCl3. The host CsPbBr3 nanorods are prepared here by B-site cation exchange in CsCdBr3 following a Cs-sublattice platform, as no such traditional protocol has been developed for their direct synthesis. In addition, direct synthesis of CdCdCl3 nanorods having an appropriate Cs-sublattice framework leading to anisotropic CsPbCl3 nanorods is also not achieved in a similar pathway. Hence, cubic CsPbCl3 nanorods are targeted from CsPbBr3 through anion exchange. However, it is observed that such anion exchange has a limiting stage beyond which the anisotropic rod is dismantled into isotropic cube structures. The chemistry of control of such limiting parameters is investigated, and the phase–shape relationship during the anion exchange is established. The key observation here for such a unique shape of CsPbBr3 is related to their orthorhombic phase, which lost its sublattice structure when the phase slowly converted to a pure cubic phase. However, with optimum Cl insertion, the mixed halide perovskite nanorods are retained, and these are also further doped with Mn(II) for obtaining anisotropic doped nanorods, and their changes in optical features are reported. Hence, the shape–phase relationship in anisotropic halide perovskite nanorods matters and controls the limit of halide exchange leading to the mixed halide perovskite anisotropic nanorods.
中文翻译:

顶点取向立方体连接图案化卤化铅钙钛矿纳米棒中的限制卤化物交换和掺杂 Mn(II)
十年来,卤化铅钙钛矿纳米晶体一直处于无机光学纳米材料的前沿。它们形成导致明亮且相位稳定的纳米晶体的化学性质也在很大程度上被理解。然而,这项研究主要集中在各向同性纳米结构上,而仅限于各向异性形状。同样,形状各向异性的研究更多地用于正交 CsPbBr3,而仅限于高带隙立方相 CsPbCl3 纳米晶体。牢记这一点,在本文中,卤化物交换在 CsPbBr3 的特定立方体连接图案化顶点导向纳米棒中进行,旨在完全转化为 CsPbCl3。在这里,宿主 CsPbBr3 纳米棒是通过在 CsCdBr3 中按照 Cs-亚晶格平台的 B 位阳离子交换制备的,因为尚未开发出用于其直接合成的此类传统方案。此外,在类似的途径中,具有适当的 Cs-亚晶格框架的 CdCdCl3 纳米棒的直接合成也无法实现,从而导致各向异性 CsPbCl3 纳米棒。因此,立方 CsPbCl3 纳米棒通过阴离子交换从 CsPbBr3 靶向。然而,据观察,这种阴离子交换有一个极限阶段,超过该阶段,各向异性棒被分解成各向同性的立方体结构。研究了控制此类限制参数的化学性质,并建立了阴离子交换过程中的相 - 形关系。对于这种独特形状的 CsPbBr3 的关键观察与它们的正交相有关,当相缓慢转化为纯立方相时,它失去了亚晶格结构。 然而,在最佳 Cl 插入下,混合卤化物钙钛矿纳米棒得以保留,并且这些纳米棒还进一步掺杂 Mn(II) 以获得各向异性掺杂纳米棒,并报道了它们在光学特性上的变化。因此,各向异性卤化物钙钛矿纳米棒中的形相关系很重要,并控制着导致混合卤化物钙钛矿各向异性纳米棒的卤化物交换极限。
更新日期:2024-12-30
中文翻译:

顶点取向立方体连接图案化卤化铅钙钛矿纳米棒中的限制卤化物交换和掺杂 Mn(II)
十年来,卤化铅钙钛矿纳米晶体一直处于无机光学纳米材料的前沿。它们形成导致明亮且相位稳定的纳米晶体的化学性质也在很大程度上被理解。然而,这项研究主要集中在各向同性纳米结构上,而仅限于各向异性形状。同样,形状各向异性的研究更多地用于正交 CsPbBr3,而仅限于高带隙立方相 CsPbCl3 纳米晶体。牢记这一点,在本文中,卤化物交换在 CsPbBr3 的特定立方体连接图案化顶点导向纳米棒中进行,旨在完全转化为 CsPbCl3。在这里,宿主 CsPbBr3 纳米棒是通过在 CsCdBr3 中按照 Cs-亚晶格平台的 B 位阳离子交换制备的,因为尚未开发出用于其直接合成的此类传统方案。此外,在类似的途径中,具有适当的 Cs-亚晶格框架的 CdCdCl3 纳米棒的直接合成也无法实现,从而导致各向异性 CsPbCl3 纳米棒。因此,立方 CsPbCl3 纳米棒通过阴离子交换从 CsPbBr3 靶向。然而,据观察,这种阴离子交换有一个极限阶段,超过该阶段,各向异性棒被分解成各向同性的立方体结构。研究了控制此类限制参数的化学性质,并建立了阴离子交换过程中的相 - 形关系。对于这种独特形状的 CsPbBr3 的关键观察与它们的正交相有关,当相缓慢转化为纯立方相时,它失去了亚晶格结构。 然而,在最佳 Cl 插入下,混合卤化物钙钛矿纳米棒得以保留,并且这些纳米棒还进一步掺杂 Mn(II) 以获得各向异性掺杂纳米棒,并报道了它们在光学特性上的变化。因此,各向异性卤化物钙钛矿纳米棒中的形相关系很重要,并控制着导致混合卤化物钙钛矿各向异性纳米棒的卤化物交换极限。































 京公网安备 11010802027423号
京公网安备 11010802027423号