当前位置:
X-MOL 学术
›
Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hemoglobin-derived amyloid fibrils: Fibrillization mechanisms and potential applications
Food Chemistry ( IF 8.5 ) Pub Date : 2024-12-29 , DOI: 10.1016/j.foodchem.2024.142671 Zerun Zhao, Di Zhao, Chunbao Li
Food Chemistry ( IF 8.5 ) Pub Date : 2024-12-29 , DOI: 10.1016/j.foodchem.2024.142671 Zerun Zhao, Di Zhao, Chunbao Li
|
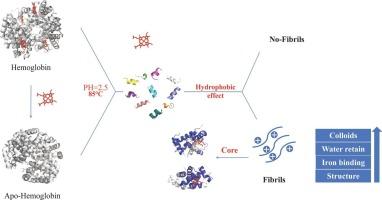
Fibrils from food proteins were widely reported but it has not been reported on sus scrofa hemoglobin. Utilizing fibrillization strategies can efficiently utilize hemoglobin and reduce waste. This work explores a new strategy to prepare hemoglobin-derived fibrils by removing the heme group. Hydrophobic interaction was found to be a key factor in promoting the fibrillization process. Heme presence hindered the fibrillization process possibly by restricting the unfolding process due to its high hydrophobicity. The core sequences of fibrils were identified as HBA 30–36, HBA 96–110, HBA 126–139, HBB 15–41, HBB 104–117 and HBB 83–145, which are generally of high hydrophobicity and HSA (Hot spots of aggregation) scores. Fibrils exhibited excellent gel properties, water retention, iron-binding properties, and enhanced positive surface charge. These findings contribute to establishing a fibrillization mechanism of hemoglobin and highlight the possibility of hemoglobin-sourced fibrils in food industry.
中文翻译:

血红蛋白来源的淀粉样蛋白原纤维:纤化机制和潜在应用
来自食物蛋白质的原纤维被广泛报道,但尚未报道 sus scrofa 血红蛋白。利用纤化策略可以有效地利用血红蛋白并减少浪费。这项工作探索了一种通过去除血红素基团来制备血红蛋白衍生的原纤维的新策略。发现疏水相互作用是促进纤化过程的关键因素。血红素的存在可能由于其高疏水性而限制了展开过程,从而阻碍了原纤化过程。原纤维的核心序列被鉴定为 HBA 30-36、HBA 96-110、HBA 126-139、HBB 15-41、HBB 104-117 和 HBB 83-145,它们通常具有高疏水性和 HSA (聚集热点) 评分。原纤维表现出优异的凝胶性能、保水性、铁结合性,并增强了表面正电荷。这些发现有助于建立血红蛋白的原纤维化机制,并强调了食品工业中血红蛋白来源的原纤维的可能性。
更新日期:2024-12-29
中文翻译:

血红蛋白来源的淀粉样蛋白原纤维:纤化机制和潜在应用
来自食物蛋白质的原纤维被广泛报道,但尚未报道 sus scrofa 血红蛋白。利用纤化策略可以有效地利用血红蛋白并减少浪费。这项工作探索了一种通过去除血红素基团来制备血红蛋白衍生的原纤维的新策略。发现疏水相互作用是促进纤化过程的关键因素。血红素的存在可能由于其高疏水性而限制了展开过程,从而阻碍了原纤化过程。原纤维的核心序列被鉴定为 HBA 30-36、HBA 96-110、HBA 126-139、HBB 15-41、HBB 104-117 和 HBB 83-145,它们通常具有高疏水性和 HSA (聚集热点) 评分。原纤维表现出优异的凝胶性能、保水性、铁结合性,并增强了表面正电荷。这些发现有助于建立血红蛋白的原纤维化机制,并强调了食品工业中血红蛋白来源的原纤维的可能性。































 京公网安备 11010802027423号
京公网安备 11010802027423号