当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible Light Photolysis at Single Atom Sites in Semiconductor Perovskite Oxides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-27 , DOI: 10.1021/jacs.4c13821 Michael G. Allan, Rachel A. Yang, Silvia Marino, Michael J. Gordon, Phillip Christopher, Eranda Nikolla
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-27 , DOI: 10.1021/jacs.4c13821 Michael G. Allan, Rachel A. Yang, Silvia Marino, Michael J. Gordon, Phillip Christopher, Eranda Nikolla
|
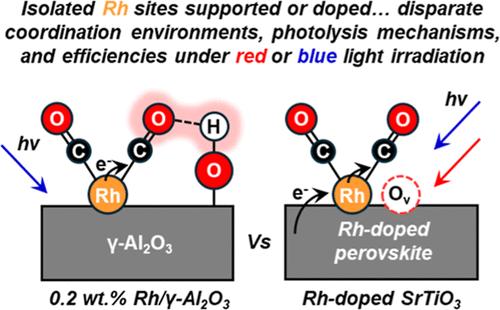
Designing catalysts with well-defined active sites with chemical functionality responsive to visible light has significant potential for overcoming scaling relations limiting chemical reactions over heterogeneous catalyst surfaces. Visible light can be leveraged to facilitate the removal of strongly bound species from well-defined single cationic sites (Rh) under mild conditions (323 K) when they are incorporated within a photoactive perovskite oxide (Rh-doped SrTiO3). CO, a key intermediate in many chemistries, forms stable geminal dicarbonyl Rh complexes (Rh+(CO)2), that could act as site blockers or poisons during a catalytic cycle. For the first time, we demonstrate that CO removal can occur at mild temperatures (323 K) under low-energy red light (635 nm) irradiation, which is not possible for supported isolated-site Rh catalysts (0.2 wt % Rh/γ-Al2O3). Photolysis of supported Rh+(CO)2 complexes (e.g., 0.2 wt % Rh/γ-Al2O3) has been demonstrated but is limited to high energy UV photons. Rigorous kinetic experiments elucidate disparate mechanisms for CO photodepletion from Rh-doped SrTiO3 and supported isolated site Rh/γ-Al2O3. CO photodepletion from supported isolated site Rh/γ-Al2O3 involves a direct metal to ligand charge transfer mechanism, whereas Rh-doped SrTiO3 is governed by electron–hole pair formation in the perovskite. We show that under visible, low-energy red light, surface Rh species in Rh-doped SrTiO3 introduce midgap energy states above the valence band that facilitate electronic excitations leading to surface CO removal. Isolated Rh sites in Rh-doped SrTiO3 also exhibit exceptional stability under multiple CO photodepletion cycles. Overall, incorporating single sites into photoactive perovskite oxides is an effective strategy to influence surface chemistries with visible light.
中文翻译:

半导体钙钛矿氧化物中单原子位点的可见光光解
设计具有明确活性位点的催化剂,这些催化剂具有对可见光有反应的化学官能团,对于克服限制非均相催化剂表面化学反应的结垢关系具有巨大潜力。当强结合物质掺入光活性钙钛矿氧化物(Rh 掺杂 SrTiO3)中时,在温和条件 (323 K) 下,可以利用可见光促进从定义明确的单个阳离子位点 (Rh) 中去除强结合物质。CO 是许多化学反应中的关键中间体,可形成稳定的双羰基二羰基 Rh 络合物 (Rh+(CO)2),在催化循环中可能充当位点阻滞剂或毒药。我们首次证明,在低能红光 (635 nm) 照射下,可以在温和 (323 K) 下进行 CO 去除,这对于负载的分离位点 Rh 催化剂 (0.2 wt % Rh/γ-Al2O3) 是不可能的。负载的 Rh+(CO)2 络合物(例如,0.2 wt % Rh/γ-Al2O3)的光解已得到证明,但仅限于高能紫外光子。严格的动力学实验阐明了 Rh 掺杂 SrTiO3 和支持分离位点 Rh/γ-Al2O3 光耗竭 CO 的不同机制。来自支持性孤立位点 Rh/γ-Al2O3 的 CO 光耗竭涉及直接金属到配体电荷转移机制,而 Rh 掺杂的 SrTiO3 受钙钛矿中电子-空穴对形成的控制。我们表明,在可见光、低能量红光下,Rh 掺杂 SrTiO3 中的表面 Rh 物质在价带上方引入中间隙能态,从而促进电子激发,从而导致表面 CO 去除。 Rh 掺杂 SrTiO3 中分离的 Rh 位点在多个 CO 光耗竭循环下也表现出优异的稳定性。总体而言,将单个位点掺入光活性钙钛矿氧化物中是用可见光影响表面化学性质的有效策略。
更新日期:2024-12-27
中文翻译:

半导体钙钛矿氧化物中单原子位点的可见光光解
设计具有明确活性位点的催化剂,这些催化剂具有对可见光有反应的化学官能团,对于克服限制非均相催化剂表面化学反应的结垢关系具有巨大潜力。当强结合物质掺入光活性钙钛矿氧化物(Rh 掺杂 SrTiO3)中时,在温和条件 (323 K) 下,可以利用可见光促进从定义明确的单个阳离子位点 (Rh) 中去除强结合物质。CO 是许多化学反应中的关键中间体,可形成稳定的双羰基二羰基 Rh 络合物 (Rh+(CO)2),在催化循环中可能充当位点阻滞剂或毒药。我们首次证明,在低能红光 (635 nm) 照射下,可以在温和 (323 K) 下进行 CO 去除,这对于负载的分离位点 Rh 催化剂 (0.2 wt % Rh/γ-Al2O3) 是不可能的。负载的 Rh+(CO)2 络合物(例如,0.2 wt % Rh/γ-Al2O3)的光解已得到证明,但仅限于高能紫外光子。严格的动力学实验阐明了 Rh 掺杂 SrTiO3 和支持分离位点 Rh/γ-Al2O3 光耗竭 CO 的不同机制。来自支持性孤立位点 Rh/γ-Al2O3 的 CO 光耗竭涉及直接金属到配体电荷转移机制,而 Rh 掺杂的 SrTiO3 受钙钛矿中电子-空穴对形成的控制。我们表明,在可见光、低能量红光下,Rh 掺杂 SrTiO3 中的表面 Rh 物质在价带上方引入中间隙能态,从而促进电子激发,从而导致表面 CO 去除。 Rh 掺杂 SrTiO3 中分离的 Rh 位点在多个 CO 光耗竭循环下也表现出优异的稳定性。总体而言,将单个位点掺入光活性钙钛矿氧化物中是用可见光影响表面化学性质的有效策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号