当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NMR Spectroscopic Study of Reaction Kinetics in Mixtures of Formaldehyde, Water, and Butynediol
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-12-27 , DOI: 10.1021/acs.iecr.4c03005
Jürgen Berje 1 , Alexander Brächer 1 , Jens Baldamus 2 , Jakob Burger 3 , Hans Hasse 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-12-27 , DOI: 10.1021/acs.iecr.4c03005
Jürgen Berje 1 , Alexander Brächer 1 , Jens Baldamus 2 , Jakob Burger 3 , Hans Hasse 1
Affiliation
|
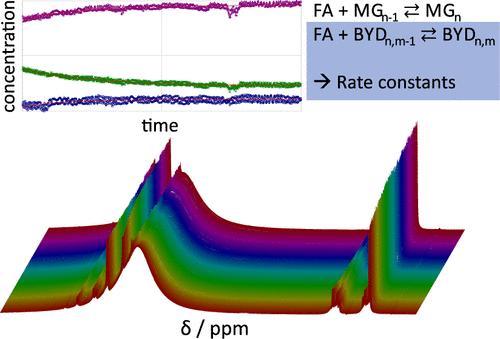
Butynediol is an important intermediate in the chemical industry and is produced from acetylene and aqueous formaldehyde. Mixtures of formaldehyde + water + butynediol are complex reacting multicomponent systems in which oligomerization reactions of formaldehyde with both water and butynediol occur. The chemical equilibria of the reactions of formaldehyde with butynediol were studied recently by quantitative NMR spectroscopy (Berje et al., DOI: 10.1002/AIC.15788). The present work complements this by a kinetic study of these reactions. Aqueous formaldehyde solutions were diluted with aqueous butynediol solutions, and the kinetic response was monitored by 1H NMR spectroscopy. Most of the experiments were carried out in NMR sample tubes; additional experiments were carried out with a micromixer NMR probe to extend the studied range to kinetics with time constants well below 1 min. The new reaction kinetic data cover temperatures between 293.15 and 328.15 K and pH values between 3 and 6. A reaction kinetic model was developed based on the equilibrium model from the previous study, and the reaction kinetic constants were determined from the new data. Furthermore, new reaction kinetic data for the system formaldehyde + water were measured, which extend the available data on that system to faster kinetics. These new data confirm the mole fraction-based reaction kinetic model of Hahnenstein et al. (DOI: 10.1021/ie00041a003).
中文翻译:

甲醛、水和丁炔二醇混合物中反应动力学的 NMR 波谱研究
丁炔二醇是化学工业的重要中间体,由乙炔和甲醛水溶液制得。甲醛 + 水 + 丁炔二醇的混合物是复杂的反应多组分系统,其中甲醛与水和丁炔二醇同时发生低聚反应。最近通过定量 NMR 波谱研究了甲醛与丁炔二醇反应的化学平衡 (Berje et al., DOI: 10.1002/AIC.15788)。目前的工作通过对这些反应的动力学研究来补充这一点。用丁炔二醇水溶液稀释甲醛水溶液,并通过 1H NMR 波谱监测动力学响应。大多数实验是在 NMR 样品管中进行的;使用微混合器 NMR 探针进行了额外的实验,以将研究范围扩展到时间常数远低于 1 分钟的动力学。新的反应动力学数据涵盖 293.15 至 328.15 K 之间的温度和 3 至 6 之间的 pH 值。基于先前研究的平衡模型开发了反应动力学模型,并根据新数据确定了反应动力学常数。此外,还测量了系统甲醛 + 水的新反应动力学数据,从而将该系统的可用数据扩展到更快的动力学。这些新数据证实了 Hahnenstein 等人基于摩尔分数的反应动力学模型 (DOI: 10.1021/ie00041a003)。
更新日期:2024-12-27
中文翻译:

甲醛、水和丁炔二醇混合物中反应动力学的 NMR 波谱研究
丁炔二醇是化学工业的重要中间体,由乙炔和甲醛水溶液制得。甲醛 + 水 + 丁炔二醇的混合物是复杂的反应多组分系统,其中甲醛与水和丁炔二醇同时发生低聚反应。最近通过定量 NMR 波谱研究了甲醛与丁炔二醇反应的化学平衡 (Berje et al., DOI: 10.1002/AIC.15788)。目前的工作通过对这些反应的动力学研究来补充这一点。用丁炔二醇水溶液稀释甲醛水溶液,并通过 1H NMR 波谱监测动力学响应。大多数实验是在 NMR 样品管中进行的;使用微混合器 NMR 探针进行了额外的实验,以将研究范围扩展到时间常数远低于 1 分钟的动力学。新的反应动力学数据涵盖 293.15 至 328.15 K 之间的温度和 3 至 6 之间的 pH 值。基于先前研究的平衡模型开发了反应动力学模型,并根据新数据确定了反应动力学常数。此外,还测量了系统甲醛 + 水的新反应动力学数据,从而将该系统的可用数据扩展到更快的动力学。这些新数据证实了 Hahnenstein 等人基于摩尔分数的反应动力学模型 (DOI: 10.1021/ie00041a003)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号