当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon, hydrogen, nitrogen and chlorine isotope fractionation during 3-chloroaniline transformation in aqueous environments by direct photolysis, TiO2 photocatalysis and hydrolysis
Water Research ( IF 11.4 ) Pub Date : 2024-12-27 , DOI: 10.1016/j.watres.2024.122956 Ning Min, Jun Yao, Hao Li, Steffen Kümmel, Thomas Schaefer, Hartmut Herrmann, Hans Hermann Richnow
Water Research ( IF 11.4 ) Pub Date : 2024-12-27 , DOI: 10.1016/j.watres.2024.122956 Ning Min, Jun Yao, Hao Li, Steffen Kümmel, Thomas Schaefer, Hartmut Herrmann, Hans Hermann Richnow
|
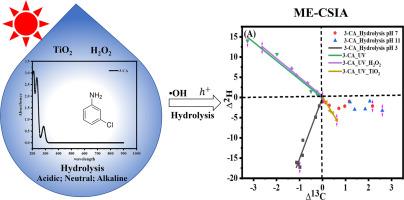
This study investigates carbon, hydrogen, nitrogen and chlorine isotope fractionation during the transformation of 3-chloroaniline (3-CA) via direct photolysis, TiO2 photocatalytic degradation at neutral condition and hydrolysis at pH 3, pH 7 and pH 11. Direct photolysis and ∙OH reaction (UV/H2 O2 ) showed similar inverse isotope fractionation (ε) for carbon (1.9 ± 0.4 ‰ and 1.9 ± 0.6 ‰), for hydrogen (6.9 ± 1.6 ‰ and 5.0 ± 2.6 ‰), and inverse chlorine (13.9 ± 3.8 ‰ and 11.9 ± 2.9 ‰) and no nitrogen isotope fractionation, respectively. In contrast, significantly different normal carbon (-0.5 ± 0.1 ‰), inverse hydrogen (6.6 ± 1.5 ‰), and normal nitrogen (-0.8 ± 0.2 ‰) and inverse chlorine (5.2 ± 3.7 ‰) isotope fractionations were observed for the photocatalysis of 3-CA by TiO2 indicating a different degradation pathway as expected from ∙OH. For hydrolysis, inverse carbon (0.7 ± 0.3 ‰) and hydrogen (12.5 ± 3.3 ‰) isotope fractionation have been found at pH 3 while a normal carbon isotope fractionation was observed at pH 7 (-0.9 ± 0.3 ‰) and pH 11 (-1.3 ± 0.4 ‰), respectively. The correlation of 2 H and 13 C, 15 N and 13 C, and 37 Cl and 13 C isotope fractionation (Λ) allowed to distinguish direct photodegradation (ΛH C = -4.6 ± 1.7 (ΛH C-YORK =-5.2 ± 1.0) and ΛCl- C = 8.7 ± 0.9 (ΛCl-C-YORK =8.0 ± 0.3)), UV/H2 O2 oxidation (ΛH C = -4.7 ± 1.0 (ΛH C-YORK =-4.5 ± 0.6) and ΛCl- C = 6.7 ± 0.8 (ΛCl-C-YORK =7.0 ± 1.0)), UV/TiO2 photocatalysis (ΛH C = -9.2 ± 3.1 (ΛH C-YORK =-9.3 ± 1.4), ΛCl-C = -10.2 ± 1.5 (ΛCl-C-YORK =-12.4 ± 1.7) and ΛN C -2.2 ± 0.3 (ΛN C-YORK =-2.3 ± 0.4)) and the modes of hydrolysis (ΛH C = 15.2 ± 5.3 (ΛH C-YORK =17.9 ± 2.9) and ΛCl- C = 0.9 ± 0.2 (ΛCl-C-YORK =1.1 ± 0.1) at pH 3) of 3-CA. The results were mechanistically interpreted highlighting the potential of CSIA to elucidate chemical oxidation and hydrolysis mechanisms of 3-CA.
中文翻译:

在水环境中通过直接光解、TiO2 光催化和水解实现 3-氯苯胺转化过程中的碳、氢、氮和氯同位素分馏
本研究研究了通过直接光解、中性条件下的 TiO2 光催化降解以及在 pH 3、pH 7 和 pH 11 下水解转化 3-氯苯胺 (3-CA) 过程中的碳、氢、氮和氯同位素分馏。直接光解和∙OH反应(UV/H2O2)对碳(1.9 ± 0.4 ‰和1.9 ± 0.6 ‰)、氢(6.9 ± 1.6 ‰和5.0 ± 2.6 ‰)和逆氯(13.9 ± 3.8 ‰和11.9 ± 2.9 ‰)和无氮同位素分馏(ε)表现出相似的逆同位素分馏(。相比之下,观察到正常碳 (-0.5 ± 0.1 ‰)、逆氢 (6.6 ± 1.5 ‰) 和正常氮 (-0.8 ± 0.2 ‰) 和逆氯 (5.2 ± 3.7 ‰) 同位素分馏的显著差异,表明与 ∙OH 不同的降解途径与预期不同。对于水解,在 pH 值为 3 时发现了反碳 (0.7 ± 0.3 ‰) 和氢 (12.5 ± 3.3 ‰) 同位素分馏,而在 pH 值为 7 (-0.9 ± 0.3 ‰) 和 pH 11 (-1.3 ± 0.4 ‰) 时分别观察到正常的碳同位素分馏。2H 和 13C、15N 和 13C 以及 37Cl 和 13C 同位素分馏 (Λ) 的相关性允许区分直接光降解 (ΛHC = -4。 6 ± 1.7 (ΛHC-YORK=-5.2 ± 1.0) 和 ΛCl-C = 8.7 ± 0.9 (ΛCl-C-YORK=8.0 ± 0.3))、UV/H2O2 氧化 (ΛHC = -4.7 ± 1.0 (ΛHC-YORK=-4.5 ± 0.6) 和 ΛCl-C = 6.7 ± 0.8 (ΛCl-C-YORK=7.0 ± 1.0))、UV/TiO2 光催化(ΛHC = -9.2 ± 3.1 (ΛHC-YORK=-9.3 ± 1.4), ΛCl-C = -10.2 ± 1.5 (ΛCl-C-YORK=-12.4 ± 1.7) 和 ΛNC -2.2 ± 0.3 (ΛNC-YORK=-2.3 ± 0.4)) 以及 3-CA 的水解模式(ΛHC = 15.2 ± 5.3 (ΛHC-YORK=17.9 ± 2.9) 和 ΛCl-C = 0.9 ± 0.2 (ΛCl-C-YORK=1.1 ± 0.1) )在 pH 值为 3 时。 对结果进行机械解释,突出了 CSIA 阐明 3-CA 化学氧化和水解机制的潜力。
更新日期:2024-12-27
中文翻译:

在水环境中通过直接光解、TiO2 光催化和水解实现 3-氯苯胺转化过程中的碳、氢、氮和氯同位素分馏
本研究研究了通过直接光解、中性条件下的 TiO2 光催化降解以及在 pH 3、pH 7 和 pH 11 下水解转化 3-氯苯胺 (3-CA) 过程中的碳、氢、氮和氯同位素分馏。直接光解和∙OH反应(UV/H2O2)对碳(1.9 ± 0.4 ‰和1.9 ± 0.6 ‰)、氢(6.9 ± 1.6 ‰和5.0 ± 2.6 ‰)和逆氯(13.9 ± 3.8 ‰和11.9 ± 2.9 ‰)和无氮同位素分馏(ε)表现出相似的逆同位素分馏(。相比之下,观察到正常碳 (-0.5 ± 0.1 ‰)、逆氢 (6.6 ± 1.5 ‰) 和正常氮 (-0.8 ± 0.2 ‰) 和逆氯 (5.2 ± 3.7 ‰) 同位素分馏的显著差异,表明与 ∙OH 不同的降解途径与预期不同。对于水解,在 pH 值为 3 时发现了反碳 (0.7 ± 0.3 ‰) 和氢 (12.5 ± 3.3 ‰) 同位素分馏,而在 pH 值为 7 (-0.9 ± 0.3 ‰) 和 pH 11 (-1.3 ± 0.4 ‰) 时分别观察到正常的碳同位素分馏。2H 和 13C、15N 和 13C 以及 37Cl 和 13C 同位素分馏 (Λ) 的相关性允许区分直接光降解 (ΛHC = -4。 6 ± 1.7 (ΛHC-YORK=-5.2 ± 1.0) 和 ΛCl-C = 8.7 ± 0.9 (ΛCl-C-YORK=8.0 ± 0.3))、UV/H2O2 氧化 (ΛHC = -4.7 ± 1.0 (ΛHC-YORK=-4.5 ± 0.6) 和 ΛCl-C = 6.7 ± 0.8 (ΛCl-C-YORK=7.0 ± 1.0))、UV/TiO2 光催化(ΛHC = -9.2 ± 3.1 (ΛHC-YORK=-9.3 ± 1.4), ΛCl-C = -10.2 ± 1.5 (ΛCl-C-YORK=-12.4 ± 1.7) 和 ΛNC -2.2 ± 0.3 (ΛNC-YORK=-2.3 ± 0.4)) 以及 3-CA 的水解模式(ΛHC = 15.2 ± 5.3 (ΛHC-YORK=17.9 ± 2.9) 和 ΛCl-C = 0.9 ± 0.2 (ΛCl-C-YORK=1.1 ± 0.1) )在 pH 值为 3 时。 对结果进行机械解释,突出了 CSIA 阐明 3-CA 化学氧化和水解机制的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号