当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Diffusion of Star and Linear Polyelectrolytes in Salt-Free and Salt Solutions
Macromolecules ( IF 5.1 ) Pub Date : 2024-12-26 , DOI: 10.1021/acs.macromol.4c01374 Aliaksei Aliakseyeu, Erica Truong, Yan-Yan Hu, Ryan Sayko, Andrey V. Dobrynin, Svetlana A. Sukhishvili
Macromolecules ( IF 5.1 ) Pub Date : 2024-12-26 , DOI: 10.1021/acs.macromol.4c01374 Aliaksei Aliakseyeu, Erica Truong, Yan-Yan Hu, Ryan Sayko, Andrey V. Dobrynin, Svetlana A. Sukhishvili
|
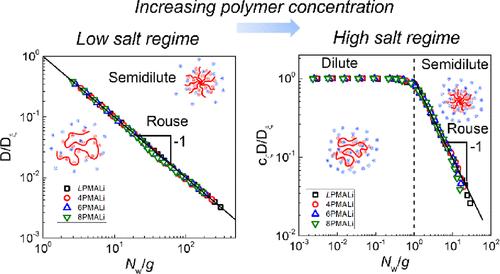
This work explored solution properties of linear and star poly(methacrylic acids) with four, six, and eight arms (LPMAA, 4PMAA, PMAA, and 8PMAA, respectively) of matched molecular weights in a wide range of pH, salt, and polymer concentrations. Experimental measurements of self-diffusion were performed by fluorescence correlation spectroscopy (FCS), and the results were interpreted using the scaling theory of polyelectrolyte solutions. While all PMAAs were pH sensitive and showed an increase in hydrodynamic radius (Rh) with pH in the dilute regime, the Rh of star polymers (measured at basic pH values) was significantly smaller for the star polyacids due to their more compact structure. Fully ionized star PMAAs were also found to be less sensitive to changes in salt concentration and type of the counterion compared to linear PMAA. While Rh of fully ionized linear PMAA decreased in the series Li+ > Na+ > K+ > Cs+ in agreement with the Hofmeister series, Rh of star PMAAs was virtually independent of type of the counterion for eight-arm PMAA. However, molecular architecture strongly affected interactions of counterions with PMAAs. In particular, 7Li NMR revealed that the spin–lattice relaxation time T1 of Li+ ions in low-salt solutions of eight-arm PMAA was ∼2-fold smaller than that in the solution of linear PMAA, suggesting slower Li+-ion dynamics within star polymers. An increase in concentration of monovalent chloride salts, cs, above that of the PMAA monomer unit concentration (cm) resulted in shrinking of both linear and star molecules, with the hydrodynamic size Rh scaling as Rh ∝ cs–0.11±0.01. Self-diffusion of linear and star polyelectrolytes was then studied in a wide range of polyelectrolyte concentrations (10–3 mol/L < cm < 0.5 mol/L) in low-salt (<10–4 mol/L of added salt) and high-salt (1 mol/L) solutions. In both the low-salt and high-salt regimes, diffusion coefficient D was lower for PMAAs with a larger number of arms at a fixed cm. In addition, in both cases, D plateaued at low polymer concentrations and decreased at higher polymer concentrations. However, while in the high-salt conditions, the concentration dependence of D reflected transitions between the dilute to semidilute solution regimes as expected for neutral chains in good and theta solvents, analysis of the diffusion data in the low-salt conditions using the scaling theory revealed a different origin of the concentration dependence of D. Specifically, in the low-salt solutions, both linear and star PMAAs exhibited unentangled (Rouse-like) dynamics in the entire range of polyelectrolyte concentrations.
中文翻译:

星形和线性聚电解质在无盐和盐溶液中的自扩散
这项工作探讨了线性和星形聚(甲基丙烯酸)的溶液特性,这些聚(甲基丙烯酸)具有 4 、 6 和 8 臂 (分别为 LPMAA、4PMAA、PMAA 和 8PMAA) 在较宽的 pH 值、盐和聚合物浓度范围内具有匹配的分子量。通过荧光相关光谱 (FCS) 进行自扩散的实验测量,并使用聚电解质溶液的缩放理论解释结果。虽然所有 PMAA 对 pH 值敏感,并且在稀态下随 pH 值显示流体动力学半径 (Rh) 增加,但由于星形多元酸的结构更紧凑,星形聚合物的 Rh(在碱性 pH 值下测量)明显较小。还发现,与线性 PMAA 相比,完全电离的星形 PMAA 对盐浓度和反离子类型的变化不太敏感。虽然全电离线性 PMAA 的 Rh 在 Li+ > Na + > K+ > Cs+ 系列中与 Hofmeister 系列一致,但星形 PMAA 的 Rh 实际上与八臂 PMAA 的对离子类型无关。然而,分子结构强烈影响反离子与 PMAA 的相互作用。特别是,7Li NMR 揭示了 Li+ 离子在八臂 PMAA 的低盐溶液中的自旋晶格弛豫时间 T 1 比线性 PMAA 溶液中的自旋晶格弛豫时间 T1 小 ∼2 倍,这表明星形聚合物内的 Li+-离子动力学较慢。 一价氯盐浓度 cs 的增加高于 PMAA 单体单位浓度 (cm) 的浓度导致线性分子和星形分子收缩,流体动力学尺寸 Rh 缩放为 Rh ∝ cs–0.11±0.01。然后,在低盐(<10-4 mol/L 添加盐)和高盐 (1 mol/L) 溶液中,研究了各种聚电解质浓度(10–3 mol/L < cm < 0.5 mol/L)中线性和星形聚电解质的自扩散。在低盐和高盐状态下,在固定 cm 下具有大量臂的 PMAA 的扩散系数 D 较低。此外,在这两种情况下,D 在低聚合物浓度下趋于稳定,而在较高聚合物浓度下下降。然而,在高盐条件下,D 的浓度依赖性反映了稀溶液到半稀溶液状态之间的转变,正如在良好和 θ 溶剂中中性链所预期的那样,而使用缩放理论对低盐条件下的扩散数据分析揭示了 D 浓度依赖性的不同来源.具体来说,在低盐溶液中,线性和星形 PMAA 在整个聚电解质浓度范围内都表现出未缠结(Rouse 样)动力学。
更新日期:2024-12-27
中文翻译:

星形和线性聚电解质在无盐和盐溶液中的自扩散
这项工作探讨了线性和星形聚(甲基丙烯酸)的溶液特性,这些聚(甲基丙烯酸)具有 4 、 6 和 8 臂 (分别为 LPMAA、4PMAA、PMAA 和 8PMAA) 在较宽的 pH 值、盐和聚合物浓度范围内具有匹配的分子量。通过荧光相关光谱 (FCS) 进行自扩散的实验测量,并使用聚电解质溶液的缩放理论解释结果。虽然所有 PMAA 对 pH 值敏感,并且在稀态下随 pH 值显示流体动力学半径 (Rh) 增加,但由于星形多元酸的结构更紧凑,星形聚合物的 Rh(在碱性 pH 值下测量)明显较小。还发现,与线性 PMAA 相比,完全电离的星形 PMAA 对盐浓度和反离子类型的变化不太敏感。虽然全电离线性 PMAA 的 Rh 在 Li+ > Na + > K+ > Cs+ 系列中与 Hofmeister 系列一致,但星形 PMAA 的 Rh 实际上与八臂 PMAA 的对离子类型无关。然而,分子结构强烈影响反离子与 PMAA 的相互作用。特别是,7Li NMR 揭示了 Li+ 离子在八臂 PMAA 的低盐溶液中的自旋晶格弛豫时间 T 1 比线性 PMAA 溶液中的自旋晶格弛豫时间 T1 小 ∼2 倍,这表明星形聚合物内的 Li+-离子动力学较慢。 一价氯盐浓度 cs 的增加高于 PMAA 单体单位浓度 (cm) 的浓度导致线性分子和星形分子收缩,流体动力学尺寸 Rh 缩放为 Rh ∝ cs–0.11±0.01。然后,在低盐(<10-4 mol/L 添加盐)和高盐 (1 mol/L) 溶液中,研究了各种聚电解质浓度(10–3 mol/L < cm < 0.5 mol/L)中线性和星形聚电解质的自扩散。在低盐和高盐状态下,在固定 cm 下具有大量臂的 PMAA 的扩散系数 D 较低。此外,在这两种情况下,D 在低聚合物浓度下趋于稳定,而在较高聚合物浓度下下降。然而,在高盐条件下,D 的浓度依赖性反映了稀溶液到半稀溶液状态之间的转变,正如在良好和 θ 溶剂中中性链所预期的那样,而使用缩放理论对低盐条件下的扩散数据分析揭示了 D 浓度依赖性的不同来源.具体来说,在低盐溶液中,线性和星形 PMAA 在整个聚电解质浓度范围内都表现出未缠结(Rouse 样)动力学。































 京公网安备 11010802027423号
京公网安备 11010802027423号