当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
FeCl2-Mediated Rearrangement of Aminoperoxides into Functionalized Tetrahydrofurans: Dynamic Non-innocence of O-Ligands at an Fe Center Coordinates a Radical Cascade
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-27 , DOI: 10.1021/jacs.4c14062 Yulia Yu. Belyakova, Peter S. Radulov, Roman A. Novikov, Ilya V. Prolomov, Nikolai V. Krivoshchapov, Michael G. Medvedev, Ivan A. Yaremenko, Igor V. Alabugin, Alexander O. Terent’ev
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-27 , DOI: 10.1021/jacs.4c14062 Yulia Yu. Belyakova, Peter S. Radulov, Roman A. Novikov, Ilya V. Prolomov, Nikolai V. Krivoshchapov, Michael G. Medvedev, Ivan A. Yaremenko, Igor V. Alabugin, Alexander O. Terent’ev
|
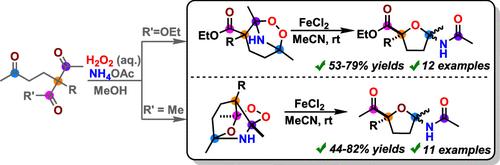
The selective reaction of cyclic aminoperoxides with FeCl2 proceeds through a sequence of O–O and C–C bond cleavages, followed by intramolecular cyclization, yielding functionalized tetrahydrofurans in 44–82% yields. Replacing the peroxyacetal group in the peroxide structure with a peroxyaminal fragment fundamentally alters the reaction pathway. Instead of producing linear functionalized ketones, this modification leads to the formation of hard-to-access substituted tetrahydrofurans. Although the aminoperoxide cores undergo multiple bond scissions, this cascade is atom-economical. Computational analysis shows that the O-ligands at the Fe center have enough radical character to promote C–C bond fragmentation and subsequent cyclization. The stereoelectronic flexibility of oxygen, combined with iron’s capacity to stabilize multiple reactive intermediates during the multistep cascade, explains the efficiency of this new atom-economic peroxide rearrangement.
中文翻译:

FeCl2 介导的氨基过氧化物重排成功能化的四氢呋喃:Fe 中心的 O 配体的动态非纯真协调自由基级联反应
环状氨基过氧化物与 FeCl2 的选择性反应经过一系列 O-O 和 C-C 键裂解,然后进行分子内环化,以 44-82% 的产率产生功能化的四氢呋喃。用过氧化物片段取代过氧化物结构中的过氧缩醛基团从根本上改变了反应途径。这种修饰不是产生线性官能化酮,而是导致难以接近的取代四氢呋喃的形成。尽管过氧化氢核心经历了多次键断裂,但这种级联是原子经济的。计算分析表明,Fe 中心的 O 配体具有足够的自由基特性,可以促进 C-C 键碎裂和随后的环化。氧的立体电子柔韧性,加上铁在多级联过程中稳定多种活性中间体的能力,解释了这种新的原子经济过氧化物重排的效率。
更新日期:2024-12-27
中文翻译:

FeCl2 介导的氨基过氧化物重排成功能化的四氢呋喃:Fe 中心的 O 配体的动态非纯真协调自由基级联反应
环状氨基过氧化物与 FeCl2 的选择性反应经过一系列 O-O 和 C-C 键裂解,然后进行分子内环化,以 44-82% 的产率产生功能化的四氢呋喃。用过氧化物片段取代过氧化物结构中的过氧缩醛基团从根本上改变了反应途径。这种修饰不是产生线性官能化酮,而是导致难以接近的取代四氢呋喃的形成。尽管过氧化氢核心经历了多次键断裂,但这种级联是原子经济的。计算分析表明,Fe 中心的 O 配体具有足够的自由基特性,可以促进 C-C 键碎裂和随后的环化。氧的立体电子柔韧性,加上铁在多级联过程中稳定多种活性中间体的能力,解释了这种新的原子经济过氧化物重排的效率。































 京公网安备 11010802027423号
京公网安备 11010802027423号