当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assembly of Pyrenes through a Quadruple Photochemical Cascade: Blocking Groups Allow Diversion from the Double Mallory Path to Photocyclization at the Bay Region
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-27 , DOI: 10.1021/jacs.4c14486 Nikolas R. Dos Santos, João Vitor Schober, Croix J. Laconsay, Alexandria M. Palazzo, Leah Kuhn, Angel Chu, Benjamin Hanks, Kenneth Hanson, Judy Wu, Igor V. Alabugin
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-27 , DOI: 10.1021/jacs.4c14486 Nikolas R. Dos Santos, João Vitor Schober, Croix J. Laconsay, Alexandria M. Palazzo, Leah Kuhn, Angel Chu, Benjamin Hanks, Kenneth Hanson, Judy Wu, Igor V. Alabugin
|
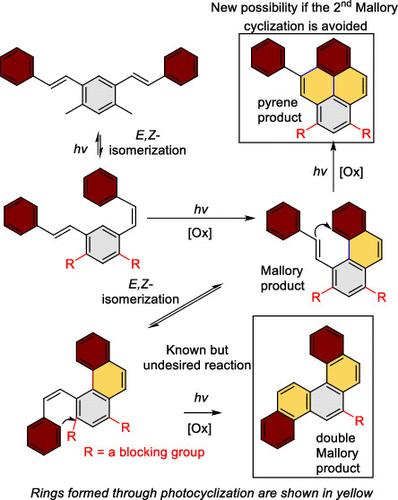
We present a six-step cascade that converts 1,3-distyrylbenzenes (bis-stilbenes) into nonsymmetric pyrenes in 40–60% yields. This sequence merges photochemical steps, E,Z-alkene isomerization, a 6π photochemical electrocyclization (Mallory photocyclization); the new bay region cyclization, with two radical iodine-mediated aromatization steps; and an optional aryl migration. This work illustrates how the inherent challenges of engineering excited state reactivity can be addressed by logical design. An unusual aspect of this cascade is that the same photochemical process (the Mallory reaction) is first promoted and then blocked in different stages within a photochemical cascade. The use of blocking groups is the key feature that makes simple bis-stilbenes suitable substrates for directed double cyclization. While the first stilbene subunit undergoes a classic Mallory photocyclization to form a phenanthrene intermediate, the next ring-forming step is diverted from the conventional Mallory path into a photocyclization of the remaining alkene at the phenanthrene’s bay region. Although earlier literature suggested that this reaction is unfavorable, we achieved this diversion via incorporation of blocking groups to prevent the Mallory photocyclization. The two photocyclizations are assisted by the relief of the excited state antiaromaticity. Reaction selectivity is controlled by substituent effects and the interplay between photochemical and radical reactivity. Furthermore, the introduction of donor substituents at the pendant styrene group can further extend this photochemical cascade through a radical 1,2-aryl migration. Rich photophysical and supramolecular properties of the newly substituted pyrenes illustrate the role of systematic variations in the structure of this classic chromophore for excited state engineering.
中文翻译:

通过四重光化学级联组装比利恩斯:阻塞基团允许从双马洛里路径转向湾区的光环化
我们提出了一个六步级联,以 40-60% 的产率将 1,3-二苯乙烯基苯 (bis-stilbenes) 转化为非对称芘。该序列合并了光化学步骤、E,Z-烯烃异构化、6π 光化学电环化(Mallory 光环化);新的湾区环化,具有两个自由基碘介导的芳构化步骤;以及可选的 aryl 迁移。这项工作说明了如何通过逻辑设计来解决工程激发态反应性的固有挑战。这种级联反应的一个不寻常之处在于,相同的光化学过程(马洛里反应)首先被促进,然后在光化学级联反应的不同阶段被阻断。封闭基团的使用是使简单的双二苯乙烯成为适合定向双环化的底物的关键特征。当第一个二苯乙烯亚基经历经典的 Mallory 光环化以形成菲中间体时,下一个环形成步骤从传统的 Mallory 路径转移到菲湾地区剩余烯烃的光环化。尽管早期的文献表明这种反应是不利的,但我们通过掺入封闭基团来防止 Mallory 光环化,从而实现了这种转移。两种光环化作用由激发态反芳香性的缓解辅助。反应选择性受取代基效应以及光化学和自由基反应性之间的相互作用控制。此外,在悬垂苯乙烯基团中引入供体取代基可以通过自由基 1,2-芳基迁移进一步扩展这种光化学级联反应。 新取代的芘丰富的光物理和超分子特性说明了这种经典发色团结构中的系统变化对激发态工程的作用。
更新日期:2024-12-27
中文翻译:

通过四重光化学级联组装比利恩斯:阻塞基团允许从双马洛里路径转向湾区的光环化
我们提出了一个六步级联,以 40-60% 的产率将 1,3-二苯乙烯基苯 (bis-stilbenes) 转化为非对称芘。该序列合并了光化学步骤、E,Z-烯烃异构化、6π 光化学电环化(Mallory 光环化);新的湾区环化,具有两个自由基碘介导的芳构化步骤;以及可选的 aryl 迁移。这项工作说明了如何通过逻辑设计来解决工程激发态反应性的固有挑战。这种级联反应的一个不寻常之处在于,相同的光化学过程(马洛里反应)首先被促进,然后在光化学级联反应的不同阶段被阻断。封闭基团的使用是使简单的双二苯乙烯成为适合定向双环化的底物的关键特征。当第一个二苯乙烯亚基经历经典的 Mallory 光环化以形成菲中间体时,下一个环形成步骤从传统的 Mallory 路径转移到菲湾地区剩余烯烃的光环化。尽管早期的文献表明这种反应是不利的,但我们通过掺入封闭基团来防止 Mallory 光环化,从而实现了这种转移。两种光环化作用由激发态反芳香性的缓解辅助。反应选择性受取代基效应以及光化学和自由基反应性之间的相互作用控制。此外,在悬垂苯乙烯基团中引入供体取代基可以通过自由基 1,2-芳基迁移进一步扩展这种光化学级联反应。 新取代的芘丰富的光物理和超分子特性说明了这种经典发色团结构中的系统变化对激发态工程的作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号