当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microtubule-Targeting NAP Peptide-Ru(II)-polypyridyl Conjugate As a Bimodal Therapeutic Agent for Triple Negative Breast Carcinoma
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-26 , DOI: 10.1021/jacs.4c11820 Atin Chatterjee, Sandip Sarkar, Sangheeta Bhattacharjee, Arpan Bhattacharyya, Surajit Barman, Uttam Pal, Raviranjan Pandey, Anitha Ethirajan, Batakrishna Jana, Benu Brata Das, Amitava Das
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-26 , DOI: 10.1021/jacs.4c11820 Atin Chatterjee, Sandip Sarkar, Sangheeta Bhattacharjee, Arpan Bhattacharyya, Surajit Barman, Uttam Pal, Raviranjan Pandey, Anitha Ethirajan, Batakrishna Jana, Benu Brata Das, Amitava Das
|
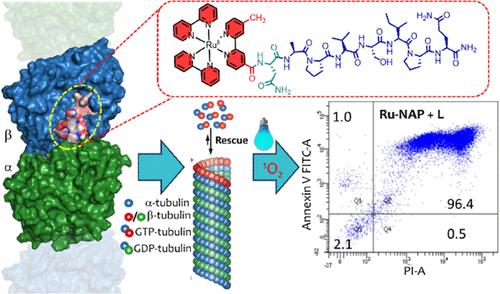
Triple-negative breast cancer (TNBC) poses significant treatment challenges due to its high metastasis, heterogeneity, and poor biomarker expression. The N-terminus of an octapeptide NAPVSIPQ (NAP) was covalently coupled to a carboxylic acid derivative of Ru(2,2′-bipy)32+ (Rubpy) to synthesize an N-stapled short peptide-Rubpy conjugate (Ru-NAP). This photosensitizer (PS) was utilized to treat TNBC through microtubule (MT) targeted chemotherapy and photodynamic therapy (PDT). Ru-NAP formed more elaborate molecular aggregates with fibrillar morphology as compared to NAP. A much higher binding affinity of Ru-NAP over NAP toward β-tubulin (KRu-NAP: (6.8 ± 0.55) × 106 M–1; KNAP: (8.2 ± 1.1) × 104 M–1) was observed due to stronger electrostatic interactions between the MT with an average linear charge density of ∼85 e/nm and the cationic Rubpy part of Ru-NAP. This was also supported by docking, simulation, and appropriate imaging studies. Ru-NAP promoted serum stability, specific binding of NAP to the E-site of the βIII-tubulin followed by the disruption of the MT network, and effective singlet oxygen generation in TNBC cells (MDA-MB-231), causing cell cycle arrest in the G2/M phase and triggering apoptosis. Remarkably, MDA-MB-231 cells were more sensitive to Ru-NAP compared to noncancerous human embryonic kidney (HEK293 cells) when exposed to light (LightIC50Ru-NAP[HEK293]: 17.2 ± 2.5 μM, compared to LightIC50Ru-NAP[MDA-MB-231]: 32.5 ± 7.8 nM, DarkIC50Ru-NAP[HEK293]: > 80 μM, compared to DarkIC50Ru-NAP[MDA-MB-231]: 2.9 ± 0.5 μM). Ru-NAP also effectively inhibited tumor growth in MDA-MB-231 xenograft models in nude mice. Our findings provide strong evidence that Ru-NAP has a potential therapeutic role in TNBC treatment.
中文翻译:

靶向微管的 NAP 肽-Ru(II)-聚吡啶基偶联物作为三阴性乳腺癌的双峰治疗剂
三阴性乳腺癌 (TNBC) 由于其高转移性、异质性和生物标志物表达差,带来了重大的治疗挑战。八肽 NAPVSIPQ (NAP) 的 N 端与 Ru(2,2′-bipy)32+ (Rubpy) 的羧酸衍生物共价偶联,合成 N -钉短肽-Rubpy 偶联物 (Ru-NAP)。这种光敏剂 (PS) 通过微管 (MT) 靶向化疗和光动力疗法 (PDT) 用于治疗 TNBC。与 NAP 相比,Ru-NAP 形成了更精细的分子聚集体,具有纤维状形态。Ru-NAP 对 β-微管蛋白 (KRu-NAP: (6.8 ± 0.55) × 106 M–1 的结合亲和力远高于 NAP; K NAP:(8.2 ± 1.1) × 104 M–1),这是由于平均线性电荷密度为 ∼85 e/nm 的 MT 与 Ru-NAP 的阳离子 Rubpy 部分之间更强的静电相互作用而观察到的。这对接、模拟和适当的成像研究也得到了支持。Ru-NAP 促进血清稳定性,NAP 与 βIII-微管蛋白 E 位点特异性结合,随后破坏 MT 网络,并在 TNBC 细胞中有效产生单线态氧 (MDA-MB-231),导致细胞周期停滞在 G2/M 期并触发细胞凋亡。值得注意的是,与非癌性人胚胎肾(HEK293 细胞)相比,MDA-MB-231 细胞在光照下对 Ru-NAP 更敏感(光IC50Ru-NAP [HEK293]:17.2 ± 2.5 μM,与光IC50Ru-NAP [MDA-MB-231]:32.5 ± 7。8 nM,深色IC50Ru-NAP[HEK293]:> 80 μM,与深色IC50Ru-NAP [MDA-MB-231]:2.9 ± 0.5 μM)相比。Ru-NAP 还有效抑制裸鼠 MDA-MB-231 异种移植模型中的肿瘤生长。我们的研究结果提供了强有力的证据,证明 Ru-NAP 在 TNBC 治疗中具有潜在的治疗作用。
更新日期:2024-12-26
中文翻译:

靶向微管的 NAP 肽-Ru(II)-聚吡啶基偶联物作为三阴性乳腺癌的双峰治疗剂
三阴性乳腺癌 (TNBC) 由于其高转移性、异质性和生物标志物表达差,带来了重大的治疗挑战。八肽 NAPVSIPQ (NAP) 的 N 端与 Ru(2,2′-bipy)32+ (Rubpy) 的羧酸衍生物共价偶联,合成 N -钉短肽-Rubpy 偶联物 (Ru-NAP)。这种光敏剂 (PS) 通过微管 (MT) 靶向化疗和光动力疗法 (PDT) 用于治疗 TNBC。与 NAP 相比,Ru-NAP 形成了更精细的分子聚集体,具有纤维状形态。Ru-NAP 对 β-微管蛋白 (KRu-NAP: (6.8 ± 0.55) × 106 M–1 的结合亲和力远高于 NAP; K NAP:(8.2 ± 1.1) × 104 M–1),这是由于平均线性电荷密度为 ∼85 e/nm 的 MT 与 Ru-NAP 的阳离子 Rubpy 部分之间更强的静电相互作用而观察到的。这对接、模拟和适当的成像研究也得到了支持。Ru-NAP 促进血清稳定性,NAP 与 βIII-微管蛋白 E 位点特异性结合,随后破坏 MT 网络,并在 TNBC 细胞中有效产生单线态氧 (MDA-MB-231),导致细胞周期停滞在 G2/M 期并触发细胞凋亡。值得注意的是,与非癌性人胚胎肾(HEK293 细胞)相比,MDA-MB-231 细胞在光照下对 Ru-NAP 更敏感(光IC50Ru-NAP [HEK293]:17.2 ± 2.5 μM,与光IC50Ru-NAP [MDA-MB-231]:32.5 ± 7。8 nM,深色IC50Ru-NAP[HEK293]:> 80 μM,与深色IC50Ru-NAP [MDA-MB-231]:2.9 ± 0.5 μM)相比。Ru-NAP 还有效抑制裸鼠 MDA-MB-231 异种移植模型中的肿瘤生长。我们的研究结果提供了强有力的证据,证明 Ru-NAP 在 TNBC 治疗中具有潜在的治疗作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号