当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Nucleophilic Amination of α-Halo Carbonyl Compounds with Free Amines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-26 , DOI: 10.1021/jacs.4c12069 Zhiyang Li, Baocheng Wang, Shuaixin Fan, Chaoshen Zhang, Jianwei Sun
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-12-26 , DOI: 10.1021/jacs.4c12069 Zhiyang Li, Baocheng Wang, Shuaixin Fan, Chaoshen Zhang, Jianwei Sun
|
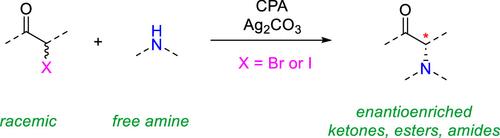
Catalytic enantioselective substitution of the readily available racemic α-halo carbonyl compounds by nitrogen nucleophiles represents one of the most convenient and direct approaches to access enantioenriched α-amino carbonyl compounds. Distinct from the two available strategies involving radicals and enolate ions, herein we have developed a new protocol featuring an electronically opposite way to weaken/cleave the carbon–halogen bond. A suitable chiral anion-based catalyst enables effective asymmetric control over the key positively charged intermediates. This protocol not only allows free amines to serve as nucleophiles but also permits different types of carbonyl compounds (ketones, esters, and amides) to participate in the enantioselective C–N bond formation, thereby providing a valuable complement to the known strategies that are limited to certain carbonyl substrates and/or nitrogen nucleophiles. Preliminary studies indicated that an SN2 pathway is operational and kinetic resolution is involved.
中文翻译:

α-卤羰基化合物与游离胺的催化对映选择性亲核胺化
氮亲核试剂催化对现成的外消旋 α-卤羰基化合物的对映体选择性取代是获得对映体富集的 α-氨基羰基化合物的最方便和最直接的方法之一。与涉及自由基和烯醇化离子的两种可用策略不同,我们在这里开发了一种新的方案,其特点是以电子相反的方式削弱/裂解碳-卤素键。合适的手性阴离子基催化剂能够对带正电荷的关键中间体进行有效的不对称控制。该方案不仅允许游离胺用作亲核试剂,还允许不同类型的羰基化合物(酮、酯和酰胺)参与对映选择性 C-N 键的形成,从而为仅限于某些羰基底物和/或氮亲核试剂的已知策略提供有价值的补充。初步研究表明,SN2 通路是可操作的,并且涉及动力学分辨率。
更新日期:2024-12-27
中文翻译:

α-卤羰基化合物与游离胺的催化对映选择性亲核胺化
氮亲核试剂催化对现成的外消旋 α-卤羰基化合物的对映体选择性取代是获得对映体富集的 α-氨基羰基化合物的最方便和最直接的方法之一。与涉及自由基和烯醇化离子的两种可用策略不同,我们在这里开发了一种新的方案,其特点是以电子相反的方式削弱/裂解碳-卤素键。合适的手性阴离子基催化剂能够对带正电荷的关键中间体进行有效的不对称控制。该方案不仅允许游离胺用作亲核试剂,还允许不同类型的羰基化合物(酮、酯和酰胺)参与对映选择性 C-N 键的形成,从而为仅限于某些羰基底物和/或氮亲核试剂的已知策略提供有价值的补充。初步研究表明,SN2 通路是可操作的,并且涉及动力学分辨率。































 京公网安备 11010802027423号
京公网安备 11010802027423号