当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Magnetic CoNi@N-Doped Carbon Composite for CO2 Electrochemical Reduction to CH4 Associated with Methanol Oxidation to Methylal in an Ionic Liquid Electrolyte
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-12-26 , DOI: 10.1021/acs.iecr.4c02973 Hongyan Li, Bairui Yang, Hui Kong, Jingxiang Zhao, Qinghai Cai
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-12-26 , DOI: 10.1021/acs.iecr.4c02973 Hongyan Li, Bairui Yang, Hui Kong, Jingxiang Zhao, Qinghai Cai
|
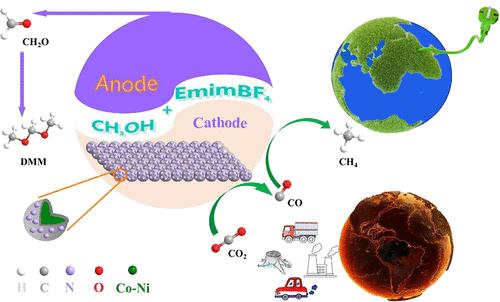
The electrochemical reduction reaction of CO2 (CO2eRR) to high value-added chemicals in an aqueous electrolyte usually faces challenges, including sluggish oxygen evolution at the anode and the competing reaction of hydrogen evolution at the cathode. A novel CoNi@NC composite with high saturation magnetization, prepared by facile pyrolysis, was used as the cathode and a Pt sheet as the anode in an ionic liquid (IL)-methanol electrolyte to conduct the CO2eRR to produce CH4 and CO, and oxidation of methanol to generate methylal (dimethoxymethane, DMM). A magnetic CoNi alloy nanomaterial (CoNi@NC) with a nitrogen-doped carbon layer-coated structure was synthesized by a one-pot method. The system achieved an associative strategy for synchronous production of high-valued chemicals via two half-reactions at the cathode and anode, respectively. The optimal CoNi@NC composite exhibited efficient catalytic activity, obtaining an average of 54.5% faradaic efficiency (FE) for DMM within 48 h and 91.9% of momentary FE for CO and CH4 at 24 h under lower energy consumption. The electrode catalyst and ionic liquid electrolyte also exhibited good recyclability and stability in the CO2eRR. Mechanism studies indicated that magnetic CoNi alloy species served as adsorption and active sites for CO2 conversion. In addition, the carbon layer coating enhanced the stability of the CoNi alloy, and N-doping introduced surface defects on the carbon layer, thereby promoting CO2 adsorption and electrocatalytic activity. Density functional theory (DFT) calculations demonstrated that the magnetic CoNi (1 1 1) species was conducive to CO2 adsorption and activation, exhibiting high selectivity for the CO2eRR.
中文翻译:

磁性 CoNi@N 掺杂碳复合材料用于 CO2 电化学还原为 CH4,与离子液体电解质中的甲醇氧化为甲基醛有关
CO2 (CO2eRR) 与水性电解质中的高附加值化学品的电化学还原反应通常面临挑战,包括阳极析氧缓慢和阴极析氢的竞争反应。在离子液体 (IL)-甲醇电解质中,以高饱和磁化强度的新型 CoNi@NC 复合材料为阴极,以 Pt 片为阳极,传导 CO2eRR 生成 CH4 和 CO,并氧化甲醇生成甲基醛(二甲氧基甲烷,DMM)。采用一锅法合成了一种具有氮掺杂碳层涂层结构的磁性 CoNi 合金纳米材料 (CoNi@NC)。该系统实现了一种结合策略,分别通过阴极和阳极的两个半反应同步生产高价值化学品。最佳 CoNi@NC 复合材料表现出高效的催化活性,在较低能耗下,DMM 在 48 h 内平均获得 54.5% 的法拉第效率 (FE),在 24 h 内获得 CO 和 CH4 的瞬时 FE 91.9%。电极催化剂和离子液体电解质在 CO2eRR 中也表现出良好的可回收性和稳定性。机理研究表明,磁性 CoNi 合金种类是 CO2 转化的吸附位点和活性位点。此外,碳层涂层增强了 CoNi 合金的稳定性,N 掺杂在碳层上引入了表面缺陷,从而促进了 CO2 的吸附和电催化活性。 密度泛函理论 (DFT) 计算表明,磁性 CoNi (1 1 1) 物质有利于 CO2 的吸附和活化,对 CO2eRR 表现出高选择性。
更新日期:2024-12-26
中文翻译:

磁性 CoNi@N 掺杂碳复合材料用于 CO2 电化学还原为 CH4,与离子液体电解质中的甲醇氧化为甲基醛有关
CO2 (CO2eRR) 与水性电解质中的高附加值化学品的电化学还原反应通常面临挑战,包括阳极析氧缓慢和阴极析氢的竞争反应。在离子液体 (IL)-甲醇电解质中,以高饱和磁化强度的新型 CoNi@NC 复合材料为阴极,以 Pt 片为阳极,传导 CO2eRR 生成 CH4 和 CO,并氧化甲醇生成甲基醛(二甲氧基甲烷,DMM)。采用一锅法合成了一种具有氮掺杂碳层涂层结构的磁性 CoNi 合金纳米材料 (CoNi@NC)。该系统实现了一种结合策略,分别通过阴极和阳极的两个半反应同步生产高价值化学品。最佳 CoNi@NC 复合材料表现出高效的催化活性,在较低能耗下,DMM 在 48 h 内平均获得 54.5% 的法拉第效率 (FE),在 24 h 内获得 CO 和 CH4 的瞬时 FE 91.9%。电极催化剂和离子液体电解质在 CO2eRR 中也表现出良好的可回收性和稳定性。机理研究表明,磁性 CoNi 合金种类是 CO2 转化的吸附位点和活性位点。此外,碳层涂层增强了 CoNi 合金的稳定性,N 掺杂在碳层上引入了表面缺陷,从而促进了 CO2 的吸附和电催化活性。 密度泛函理论 (DFT) 计算表明,磁性 CoNi (1 1 1) 物质有利于 CO2 的吸附和活化,对 CO2eRR 表现出高选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号