当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Exploration of the Competitive Mechanism for Hydrodehydration and Decarboxylation of 2,5-Furandicarboxylic Acid by Pt1 and Pt3 Supported on Nb2O5
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-12-26 , DOI: 10.1021/acs.jpcc.4c06339 Ting-Hao Liu, Shuai Fu, Jin-Tao Gou, Ming-Hui Zhang, Chang-Wei Hu, Hua-Qing Yang
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-12-26 , DOI: 10.1021/acs.jpcc.4c06339 Ting-Hao Liu, Shuai Fu, Jin-Tao Gou, Ming-Hui Zhang, Chang-Wei Hu, Hua-Qing Yang
|
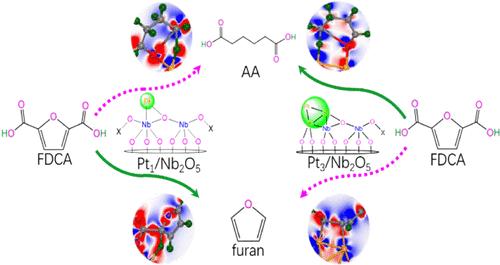
How the size of the Pt-cluster over Nb2O5 affects the catalytic performance for the hydrodehydration of 2,5-furandicarboxylic acid (FDCA) is not yet clear at the molecular level. We rationally designed the Pt1/Nb2O5 ([Pt1]) and Pt3/Nb2O5 ([Pt3]) catalyst models. Over them, the catalytic mechanism for the hydrodehydration of FDCA to adipic acid (AA) has been theoretically investigated in aqueous solution at the GGA-PBE/DNP level together with its side reactions. The hydrodehydration of FDCA to AA is associated with the ring-opening of furan as the rate-determining step, whereas the decarboxylation of FDCA to furan is related to the cleavage of the C–C bond as the rate-determining step. For the conversion of FDCA, [Pt3] shows higher catalytic activity than [Pt1], because of the less positive charge of Pt1–Pt2 dual-sites over [Pt3] than that of the Pt1 single-site over [Pt1]. Here, the Pt1–Pt2 dual-sites over [Pt3] have a more important synergistic effect on the cleavage of both C5–O3 and C5–C6 bonds compared with the Pt single-site over [Pt1]. Furthermore, [Pt3] selectively favors the FDCA-to-AA hydrodehydration, whereas [Pt1] selectively promotes the FDCA-to-furan decarboxylation. The current research results should provide some theoretical clues for designing novel supported cluster metal oxide catalysts for the hydrogenation of biomass.
中文翻译:

Nb2O5 上 Pt1 和 Pt3 负载对 2,5-呋喃二羧酸加氢脱水脱羧的竞争机制的理论探索
Nb2O5 上的 Pt 簇大小如何影响 2,5-呋喃二羧酸 (FDCA) 加氢脱水的催化性能在分子水平上尚不清楚。我们合理设计了 Pt1/Nb2O5 ([Pt1]) 和 Pt3/Nb2O5 ([Pt3]) 催化剂模型。在它们之上,FDCA 加氢脱水为己二酸 (AA) 的催化机制已在 GGA-PBE/DNP 水平的水溶液及其副反应中进行了理论研究。FDCA 脱水为 AA 与作为速率确定步骤的呋喃开环有关,而 FDCA 脱羧为呋喃与作为速率决定步骤的 C-C 键裂解有关。对于 FDCA 的转化,[Pt3] 显示出比 [Pt1] 更高的催化活性,因为 Pt1-Pt2 双位点在 [Pt3] 上的正电荷比 Pt1 单位点在 [Pt1] 上的正电荷小。在这里,与 [Pt1] 上的 Pt 单位点相比,[Pt3] 上的 Pt1-Pt2 双位点对 C5-O3 和 C5-C6 键的切割具有更重要的协同作用。此外,[Pt3] 选择性地促进 FDCA 到 AA 的加氢脱水,而 [Pt1] 选择性地促进 FDCA 到呋喃的脱羧。目前的研究结果为设计用于生物质加氢的新型负载型团簇金属氧化物催化剂提供一些理论线索。
更新日期:2024-12-26
中文翻译:

Nb2O5 上 Pt1 和 Pt3 负载对 2,5-呋喃二羧酸加氢脱水脱羧的竞争机制的理论探索
Nb2O5 上的 Pt 簇大小如何影响 2,5-呋喃二羧酸 (FDCA) 加氢脱水的催化性能在分子水平上尚不清楚。我们合理设计了 Pt1/Nb2O5 ([Pt1]) 和 Pt3/Nb2O5 ([Pt3]) 催化剂模型。在它们之上,FDCA 加氢脱水为己二酸 (AA) 的催化机制已在 GGA-PBE/DNP 水平的水溶液及其副反应中进行了理论研究。FDCA 脱水为 AA 与作为速率确定步骤的呋喃开环有关,而 FDCA 脱羧为呋喃与作为速率决定步骤的 C-C 键裂解有关。对于 FDCA 的转化,[Pt3] 显示出比 [Pt1] 更高的催化活性,因为 Pt1-Pt2 双位点在 [Pt3] 上的正电荷比 Pt1 单位点在 [Pt1] 上的正电荷小。在这里,与 [Pt1] 上的 Pt 单位点相比,[Pt3] 上的 Pt1-Pt2 双位点对 C5-O3 和 C5-C6 键的切割具有更重要的协同作用。此外,[Pt3] 选择性地促进 FDCA 到 AA 的加氢脱水,而 [Pt1] 选择性地促进 FDCA 到呋喃的脱羧。目前的研究结果为设计用于生物质加氢的新型负载型团簇金属氧化物催化剂提供一些理论线索。































 京公网安备 11010802027423号
京公网安备 11010802027423号