当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic Insights into the Acid Strength of Sulfonate Linkers in Confined Space
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-12-25 , DOI: 10.1021/acs.jpcc.4c06072 Gourav Shrivastav, Jasmin Kaur, M. Ali Haider
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-12-25 , DOI: 10.1021/acs.jpcc.4c06072 Gourav Shrivastav, Jasmin Kaur, M. Ali Haider
|
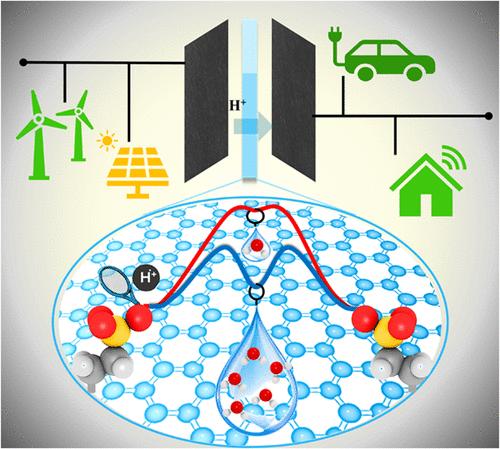
Sulfonic acid groups exhibit a strong ability to deprotonate in aqueous environments and facilitate proton conduction through the membrane. However, the membrane’s hydration level is a critical factor influencing both the acid strength and the efficiency of proton conduction mechanisms. In this study, we employ ab initio molecular dynamics (AIMD) simulations augmented with metadynamics to explore the thermodynamic drivers governing the acid strength, highlighting the complex interactions between solvent molecules and functional groups within confinement at different interlinker spacings. Our computational studies reveal that a minimum of four water molecules are required to deprotonate these ethyl sulfonic acid (ESA) units, while internal energy drives the transition. For these ESA units, the tendency to accept and donate acidic protons is correlated to the dynamic behavior of hydronium ions. The distribution of a hydronium ion in the vicinity of ESA units is also found to be more sensitive to interlinker spacing than the water content itself. Therefore, the interplay of interlinker spacing and hydration level is crucial to further optimize the application of these systems, thereby facilitating the development of next-generation technologies in fields such as heterogeneous catalysis, proton exchange membranes, and energy conversion systems.
中文翻译:

密闭空间内磺酸盐连接剂酸强度的热力学见解
磺酸基团在水性环境中表现出很强的去质子化能力,并促进质子通过膜传导。然而,膜的水合作用水平是影响酸强度和质子传导机制效率的关键因素。在这项研究中,我们采用从头计算分子动力学 (AIMD) 模拟,并辅以元动力学来探索控制酸强度的热力学驱动因素,突出了溶剂分子和官能团在不同交联剂间距下的限制内之间的复杂相互作用。我们的计算研究表明,至少需要四个水分子才能使这些乙基磺酸 (ESA) 单元去质子化,而内能驱动转变。对于这些 ESA 单元,接受和供体酸性质子的趋势与水合氢离子的动态行为相关。还发现水合氢离子在 ESA 单元附近的分布比含水量本身对互连剂间距更敏感。因此,交联剂间距和水合水平的相互作用对于进一步优化这些系统的应用至关重要,从而促进多相催化、质子交换膜和能量转换系统等领域的下一代技术的发展。
更新日期:2024-12-26
中文翻译:

密闭空间内磺酸盐连接剂酸强度的热力学见解
磺酸基团在水性环境中表现出很强的去质子化能力,并促进质子通过膜传导。然而,膜的水合作用水平是影响酸强度和质子传导机制效率的关键因素。在这项研究中,我们采用从头计算分子动力学 (AIMD) 模拟,并辅以元动力学来探索控制酸强度的热力学驱动因素,突出了溶剂分子和官能团在不同交联剂间距下的限制内之间的复杂相互作用。我们的计算研究表明,至少需要四个水分子才能使这些乙基磺酸 (ESA) 单元去质子化,而内能驱动转变。对于这些 ESA 单元,接受和供体酸性质子的趋势与水合氢离子的动态行为相关。还发现水合氢离子在 ESA 单元附近的分布比含水量本身对互连剂间距更敏感。因此,交联剂间距和水合水平的相互作用对于进一步优化这些系统的应用至关重要,从而促进多相催化、质子交换膜和能量转换系统等领域的下一代技术的发展。































 京公网安备 11010802027423号
京公网安备 11010802027423号