当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal Conversion of Calcium Sulfate Hemihydrate in the H2SO4–H3PO4–H2SiF6 System
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-12-26 , DOI: 10.1021/acs.iecr.4c04068 Xingmin Peng, Ganyu Zhu, Huiquan Li, Xiaodan Su, Ziheng Meng, Wenjuan Zhang, Dehua Xu, Zuohua Liu, Qian Zhang
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-12-26 , DOI: 10.1021/acs.iecr.4c04068 Xingmin Peng, Ganyu Zhu, Huiquan Li, Xiaodan Su, Ziheng Meng, Wenjuan Zhang, Dehua Xu, Zuohua Liu, Qian Zhang
|
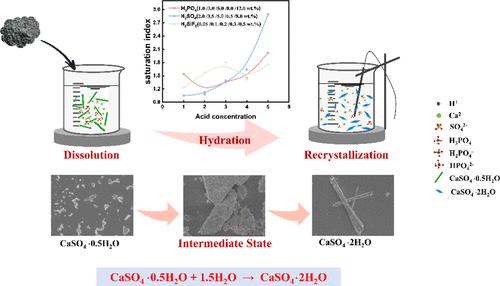
The byproduct gypsum from the dihydrate wet-process phosphoric acid production presents a complex composition that limits its potential for high-value utilization. The hemihydrate–dihydrate (HH–DH) process produces concentrated phosphoric acid while reducing gypsum impurities through a two-step process involving hemihydrate reaction and recrystallization. Crucially, residual acids from the hemihydrate stage may influence the recrystallization step. Here, we systematically investigate the effects of H3PO4, H2SO4, and H2SiF6 on the hydration kinetics and morphology of CaSO4·2H2O during this process. Our results showed that over a range of acid concentrations, an increase in the concentration of H3PO4 delays the conversion of CaSO4·0.5H2O, H2SO4 accelerates the conversion, and H2SiF6 has a negligible effect on the hydration rate. The presence of these acids changed the supersaturation of the solution, impacting the crystallization of CaSO4·2H2O. Furthermore, both H2SO4 and H2SiF6 increase crystal defects and reduce crystalline grain size, and decreasing the concentrations of H2SO4 and H2SiF6 in gypsum hemihydrate resulted in crystals with large aspect ratios. This study provides detailed insights into the hydration mechanism of the HH–DH process, which proceeds by a dissolution–recrystallization mechanism, elucidating the role of residual acid, and provides guidance for the control of conditions in the hemihydrate conversion process based on the effect of acid concentration, which provides valuable guidance for optimizing production.
中文翻译:

H2SO4–H3PO4–H2SiF6 系统中硫酸钙半水合物的晶体转化
二水合物湿法磷酸生产产生的副产品石膏具有复杂的成分,限制了其高价值利用的潜力。半水合物-二水合物 (HH-DH) 工艺通过涉及半水合物反应和重结晶的两步工艺生产浓磷酸,同时减少石膏杂质。至关重要的是,半水合物阶段的残留酸可能会影响重结晶步骤。在这里,我们系统研究了 H3PO4、H2SO4 和 H2SiF6 在此过程中对 CaSO4·2H2O 水化动力学和形态的影响。我们的结果表明,在一定酸浓度范围内,H3PO4 浓度的增加会延迟 CaSO4·0.5H2O 的转化,H2SO4 会加速转化,而 H2SiF6 对水化速率的影响可以忽略不计。这些酸的存在改变了溶液的过饱和度,影响了 CaSO4·2H2O 的结晶。此外,H2SO4 和 H2SiF6 都会增加晶体缺陷并减小晶粒尺寸,降低石膏半水合物中 H2SO4 和 H2SiF6 的浓度会导致晶体具有较大的纵横比。 本研究详细介绍了 HH-DH 过程的水合机理,该过程通过溶解-重结晶机制进行,阐明了残酸的作用,并为基于酸浓度影响的半水合物转化过程的条件控制提供了指导,为优化生产提供了有价值的指导。
更新日期:2024-12-26
中文翻译:

H2SO4–H3PO4–H2SiF6 系统中硫酸钙半水合物的晶体转化
二水合物湿法磷酸生产产生的副产品石膏具有复杂的成分,限制了其高价值利用的潜力。半水合物-二水合物 (HH-DH) 工艺通过涉及半水合物反应和重结晶的两步工艺生产浓磷酸,同时减少石膏杂质。至关重要的是,半水合物阶段的残留酸可能会影响重结晶步骤。在这里,我们系统研究了 H3PO4、H2SO4 和 H2SiF6 在此过程中对 CaSO4·2H2O 水化动力学和形态的影响。我们的结果表明,在一定酸浓度范围内,H3PO4 浓度的增加会延迟 CaSO4·0.5H2O 的转化,H2SO4 会加速转化,而 H2SiF6 对水化速率的影响可以忽略不计。这些酸的存在改变了溶液的过饱和度,影响了 CaSO4·2H2O 的结晶。此外,H2SO4 和 H2SiF6 都会增加晶体缺陷并减小晶粒尺寸,降低石膏半水合物中 H2SO4 和 H2SiF6 的浓度会导致晶体具有较大的纵横比。 本研究详细介绍了 HH-DH 过程的水合机理,该过程通过溶解-重结晶机制进行,阐明了残酸的作用,并为基于酸浓度影响的半水合物转化过程的条件控制提供了指导,为优化生产提供了有价值的指导。































 京公网安备 11010802027423号
京公网安备 11010802027423号