当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon Dots with Polycyclic Dipeptide Structure Surpass Natural Hydrolase Performance
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-12-26 , DOI: 10.1002/adfm.202423470 Mengling Zhang, Zeyu Wu, Yan Zhang, Tao Hu, Wenwen Li, Hui Huang, Mingwang Shao, Yang Liu, Xing Fan, Zhenhui Kang
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-12-26 , DOI: 10.1002/adfm.202423470 Mengling Zhang, Zeyu Wu, Yan Zhang, Tao Hu, Wenwen Li, Hui Huang, Mingwang Shao, Yang Liu, Xing Fan, Zhenhui Kang
|
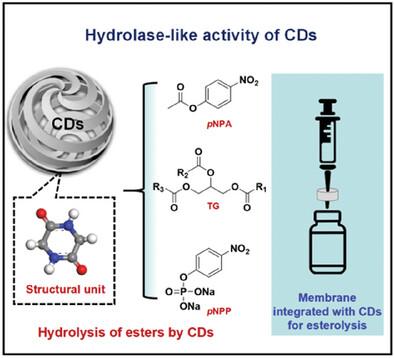
Nature enzymes always suffer from high costs, harsh conditions, and instability, while, artificial one offers possibilities for addressing current challenges and plays a significant role in future industrial production. Here, the carbon dots (CDs), with well‐defined polycyclic dipeptide structures, are reported to exhibit superior hydrolase‐like catalytic performance beyond those of natural enzymes. Compared to natural lipase, the obtained CDs not only exhibit superior catalytic activity (2.85 times increase in Vm ) but also have a broader range of substrate concentrations (0–8.0 mm ) and substrate types (fatty ester, aromatic ester, phosphate ester), along with simpler catalytic conditions and superior stability. The highly efficient catalytic activity of CDs is attributed to the low activation energy of the reaction (Ea : 13.74 kJ mol−1 ) and strong adsorption to the substrates. The results of theoretical calculations and comparative experiments demonstrate that the adsorption sites of CDs are located on the nitrogen atoms of the polycyclic dipeptide. Furthermore, a membrane integrated with CDs (membrane‐catalyst) is constructed, through which the instantaneous hydrolysis of esters can be realized. This work provides new insight into the enzyme‐like catalytic mechanisms of CDs and offers a new approach to designing enzyme‐like materials with high performance.
中文翻译:

具有多环二肽结构的碳点优于天然水解酶性能
天然酶总是成本高、条件恶劣和不稳定,而人工酶为应对当前挑战提供了可能性,并在未来的工业生产中发挥着重要作用。据报道,在这里,具有明确多环二肽结构的碳点 (CDs) 表现出优于天然酶的类似水解酶的催化性能。与天然脂肪酶相比,获得的 CDs 不仅表现出优异的催化活性(Vm 增加 2.85 倍),而且具有更广泛的底物浓度范围 (0-8.0 mm) 和底物类型(脂肪酯、芳香酯、磷酸酯),以及更简单的催化条件和卓越的稳定性。CDs 的高效催化活性归因于反应的低活化能 (Ea: 13.74 kJ mol-1) 和对底物的强烈吸附。理论计算和比较实验结果表明,CDs 的吸附位点位于多环二肽的氮原子上。此外,构建了与 CDs (膜-催化剂) 集成的膜,通过该膜可以实现酯的瞬时水解。这项工作为 CDs 的类酶催化机制提供了新的见解,并为设计高性能的类酶材料提供了一种新方法。
更新日期:2024-12-26
中文翻译:

具有多环二肽结构的碳点优于天然水解酶性能
天然酶总是成本高、条件恶劣和不稳定,而人工酶为应对当前挑战提供了可能性,并在未来的工业生产中发挥着重要作用。据报道,在这里,具有明确多环二肽结构的碳点 (CDs) 表现出优于天然酶的类似水解酶的催化性能。与天然脂肪酶相比,获得的 CDs 不仅表现出优异的催化活性(Vm 增加 2.85 倍),而且具有更广泛的底物浓度范围 (0-8.0 mm) 和底物类型(脂肪酯、芳香酯、磷酸酯),以及更简单的催化条件和卓越的稳定性。CDs 的高效催化活性归因于反应的低活化能 (Ea: 13.74 kJ mol-1) 和对底物的强烈吸附。理论计算和比较实验结果表明,CDs 的吸附位点位于多环二肽的氮原子上。此外,构建了与 CDs (膜-催化剂) 集成的膜,通过该膜可以实现酯的瞬时水解。这项工作为 CDs 的类酶催化机制提供了新的见解,并为设计高性能的类酶材料提供了一种新方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号