当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coupling Anionic Oxygen Redox with Selenium for Stable High‐Voltage Sodium Layered Oxide Cathodes
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-12-26 , DOI: 10.1002/adfm.202417758 Zhichen Xue, Neha Bothra, Dechao Meng, Guangxia Feng, Yuqi Li, Tony Cui, Hongchang Hao, Sang‐Jun Lee, Yijin Liu, Michal Bajdich, Jagjit Nanda, Xueli Zheng
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-12-26 , DOI: 10.1002/adfm.202417758 Zhichen Xue, Neha Bothra, Dechao Meng, Guangxia Feng, Yuqi Li, Tony Cui, Hongchang Hao, Sang‐Jun Lee, Yijin Liu, Michal Bajdich, Jagjit Nanda, Xueli Zheng
|
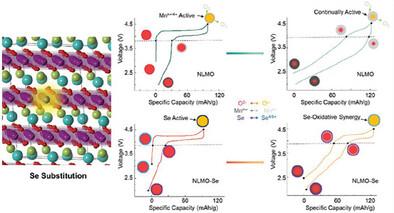
Utilizing anion redox reaction is crucial for developing the next generation of high‐energy density, low‐cost sodium‐ion batteries. However, the irreversible oxygen redox reaction in Na‐ion layered cathodes, which leads to voltage fading and reduced overall lifespan, has hindered their practical application. In this study, selenium is incorporated as a synergistic redox active center of oxygen to improve the stability of Na‐ion cathodes. The redesigned cathode maintains stable voltage by demonstrating reversible oxygen redox while significantly suppressing the redox activity of manganese. The anionic redox contribution capacity of the selenium‐doped Na0.6 Li0.2 Mn0.8 O2 cathode remains as high as 84% after 50 cycles, while the pristine Na0.6 Li0.2 Mn0.8 O2 cathode experiences a reduction to 39% of its initial capacity. The X‐ray photoelectron spectroscopy data and computational analysis further revealed that selenium doping participates in redox as Se+4/5 which stabilizes the charged state and increases the energy step for O─O dimerization, thus improving the stability and lifespan of Na0.6 Li0.2 Mn0.8 O2 cathodes. The findings highlight the potential of redox coupling design to address the issue of voltage fade caused by irreversible anionic redox.
中文翻译:

阴离子氧氧化还原与硒耦合,形成稳定的高压钠层状氧化物阴极
利用阴离子氧化还原反应对于开发下一代高能量密度、低成本钠离子电池至关重要。然而,Na-ion 层状阴极中不可逆的氧氧化还原反应导致电压衰减并缩短整体寿命,阻碍了它们的实际应用。在这项研究中,硒被掺入作为氧的协同氧化还原活性中心,以提高 Na-ion 阴极的稳定性。重新设计的阴极通过展示可逆氧氧化还原来保持稳定的电压,同时显着抑制锰的氧化还原活性。硒掺杂 Na0.6Li0.2Mn0.8O2 阴极的阴离子氧化还原贡献容量在 50 次循环后仍高达 84%,而原始 Na0.6Li0.2Mn0.8O2 阴极的阴离子氧化还原贡献容量降低至其初始容量的 39%。X 射线光电子能谱数据和计算分析进一步表明,硒掺杂以 Se+4/5 的形式参与氧化还原,稳定了带电状态并增加了 O−O 二聚化的能量步长,从而提高了 Na0.6Li0.2Mn0.8O2 阴极的稳定性和寿命。研究结果强调了氧化还原耦合设计在解决不可逆阴离子氧化还原引起的电压衰退问题方面的潜力。
更新日期:2024-12-26
中文翻译:

阴离子氧氧化还原与硒耦合,形成稳定的高压钠层状氧化物阴极
利用阴离子氧化还原反应对于开发下一代高能量密度、低成本钠离子电池至关重要。然而,Na-ion 层状阴极中不可逆的氧氧化还原反应导致电压衰减并缩短整体寿命,阻碍了它们的实际应用。在这项研究中,硒被掺入作为氧的协同氧化还原活性中心,以提高 Na-ion 阴极的稳定性。重新设计的阴极通过展示可逆氧氧化还原来保持稳定的电压,同时显着抑制锰的氧化还原活性。硒掺杂 Na0.6Li0.2Mn0.8O2 阴极的阴离子氧化还原贡献容量在 50 次循环后仍高达 84%,而原始 Na0.6Li0.2Mn0.8O2 阴极的阴离子氧化还原贡献容量降低至其初始容量的 39%。X 射线光电子能谱数据和计算分析进一步表明,硒掺杂以 Se+4/5 的形式参与氧化还原,稳定了带电状态并增加了 O−O 二聚化的能量步长,从而提高了 Na0.6Li0.2Mn0.8O2 阴极的稳定性和寿命。研究结果强调了氧化还原耦合设计在解决不可逆阴离子氧化还原引起的电压衰退问题方面的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号