American Journal of Hematology ( IF 10.1 ) Pub Date : 2024-12-26 , DOI: 10.1002/ajh.27570 Patricia Carey, Animesh Pardanani, Patrick Starlinger, Ayalew Tefferi, Naseema Gangat
|
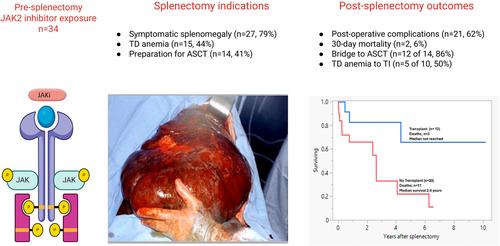
The management of symptomatic splenomegaly in myelofibrosis (MF) was revolutionized by the discovery of the JAK2V617F mutation and subsequent development of JAK2 inhibitors (JAKi) [1-4]. Currently, four JAKi are approved by the Food and Drug Administration (FDA) for treatment of MF: ruxolitinib, fedratinib, pacritinib, and momelotinib which alleviate symptomatic splenomegaly and constitutional symptoms. In the COMFORT I (ruxolitinib vs. placebo), and COMFORT II studies (ruxolitinib vs. best available therapy), spleen volume reduction of > 35% was achieved in 41.9% and 28% of ruxolitinib-treated patients, respectively [5, 6]. However, the benefits of JAKi therapy are often transient, and in the COMFORT-I and II trials, 55% and 75% of patients had discontinued ruxolitinib at 3-year and 5-year follow-up, respectively [4, 7, 8]. We have previously shown that patients with ruxolitinib-resistant splenomegaly derive limited benefit from switching to fedratinib [9], and involved-field radiotherapy or splenectomy is often advised as a bridge to allogeneic stem cell transplant (ASCT) [2, 10]. In this study, we describe the outcomes of 34 patients with MF that underwent splenectomy after exposure to JAKi therapy.
After institutional board review approval, study patients were identified from the Mayo Clinic (USA) clinical database based on the following criteria: (i) diagnosis of MF according to the International Consensus Classification [11]; (ii) documentation of JAKi therapy exposure followed by splenectomy; (iii) availability of information before and after splenectomy. We identified 39 patients with prior JAKi treatment. Two patients were excluded due to primary indication for splenectomy, with one undergoing splenectomy due to trauma and the other for metastatic solid tumor. Three patients were excluded due to lack of follow-up information after splenectomy. Ultimately, 34 patients were included who underwent an open (n = 29) or laparoscopic (n = 5) splenectomy between 2011 and 2024. Transfusion independence was defined as more than 12 weeks without a red blood cell transfusion and hemoglobin ≥ 1.5 g/dL. [12] Major bleeding event was defined by criteria from the International Society on Thrombosis and Haemostasis [13]. Clinical and laboratory variables at the time of MF diagnosis, at splenectomy, and 3 and 12 months post-procedure were collected. Conventional statistical methods were employed for data analysis (JMP Pro 17.0.0, SAS Institute, Cary, NC, USA).
A total of 34 patients with MF (primary MF [PMF, n = 18], post-polycythemia vera MF [post-PV MF, n = 15], and post-essential thrombocythemia MF [post-ET MF, n = 1]); median age; 63 years, 59% males, median spleen size; 25.1 cm (14.7–36) who underwent splenectomy following treatment with one or more JAKi were included. Baseline characteristics and prior JAKi exposure at the time of splenectomy are outlined in Table S1. JAK2V617F mutation was detected in 21 (62%), CALR mutation in 9 (26%), MPL in 1 (3%) case, while 3 (9%) were triple-negative. The majority of patients had received one JAKi (n = 26; 76%), six (18%) received two, and two (6%) patients received three JAKi. Specific JAKi included ruxolitinib in 27 (79%), momelotinib in 5 (15%), fedratinib in 3 (9%), pacritinib in 3 (9%), and BMS 911543 JAKi as part of a clinical trial in 5 (15%) patients. Prior treatment also included splenic radiation in 4 patients (median 0.6 years [0.2–1.2] before splenectomy); preoperative splenic embolization was performed in 4 patients; one patient underwent embolization 10 months prior to splenectomy. Median interval from MF diagnosis to splenectomy was 10.3 years (1.0–35.4) and from initiation of JAKi therapy to splenectomy was 1.8 years (0.03–12.1). Most common indications for splenectomy included symptomatic splenomegaly in 27 (79%) patients, transfusion-dependent anemia in 15 (44%), and in preparation for ASCT in 14 (41%) patients. Overall, 23 (68%) patients discontinued JAKi before splenectomy, whereas the remaining 11 patients continued on JAKi during/after surgery.
中文翻译:

JAK2 抑制剂治疗骨髓纤维化患者脾切除术的结果:梅奥诊所连续 34 例的经验
随着 JAK2V617F 突变的发现和随后的 JAK2 抑制剂 (JAKi) 的发展,骨髓纤维化 (MF) 症状性脾肿大的管理发生了革命性的变化 [1-4]。目前,美国食品药品监督管理局 (FDA) 批准了四种 JAKi 用于治疗 MF:ruxolitinib、fedratinib、pacritinib 和莫莫替尼,可缓解症状性脾肿大和全身症状。在 COMFORT I (ruxolitinib vs. 安慰剂) 和 COMFORT II 研究 (ruxolitinib vs. 最佳可用疗法) 中,41.9% 和 28% 的 ruxolitinib 治疗患者的脾脏体积分别减少了 > 35% [5, 6]。然而,JAKi 疗法的益处通常是短暂的,在 COMFORT-I 和 II 试验中,分别有 55% 和 75% 的患者在 3 年和 5 年随访时停用了 ruxolitinib [4, 7, 8]。我们之前已经表明,芦可替尼耐药性脾肿大患者改用 fedratinib 的益处有限 [9],并且通常建议将受累区域放疗或脾切除术作为通向同种异体干细胞移植 (ASCT) 的过渡 [2, 10]。在这项研究中,我们描述了 34 例 MF 患者在接受 JAKi 治疗后接受脾切除术的结果。
在机构委员会审查批准后,根据以下标准从梅奥诊所(美国)临床数据库中确定了研究患者:(i) 根据国际共识分类 [11] 诊断为 MF;(ii) 脾切除术后 JAKi 治疗暴露的记录;(iii) 脾切除术前后信息的可用性。我们确定了 39 例既往接受过 JAKi 治疗的患者。2 例患者因脾切除术的主要指征而被排除,其中 1 例因外伤接受脾切除术,另一例因转移性实体瘤。3 例患者因脾切除术后缺乏随访信息而被排除在外。最终,纳入了 34 例在 2011 年至 2024 年间接受开放 (n = 29) 或腹腔镜 (n = 5) 脾切除术的患者。不依赖输血定义为超过 12 周未输注红细胞且血红蛋白≥ 1.5 g/dL。[12] 大出血事件由国际血栓形成和止血学会的标准定义 [13]。收集 MF 诊断时、脾切除术时以及术后 3 个月和 12 个月的临床和实验室变量。采用常规统计方法进行数据分析(JMP Pro 17.0.0,SAS Institute,Cary,NC,USA)。
共有 34 例 MF 患者 (原发性 MF [PMF, n = 18]、真性红细胞增多症后 MF [PV 后 MF, n = 15] 和原发性血小板增多症后 MF [ET 后 MF, n = 1]);中位年龄;63 岁,59% 男性,脾脏中位大小;包括 25.1 cm (14.7-36) 在接受一个或多个 JAKi 治疗后接受脾切除术。表 S1 概述了脾切除术时的基线特征和既往 JAKi 暴露。21 例 (62%) 检测到 JAK2V617F 突变,9 例 (26%) 检测到 CALR 突变,1 例 (3%) 检测到 MPL,而 3 例 (9%) 为三阴性。大多数患者接受了 1 次 JAKi (n = 26;76%),6 例 (18%) 接受了 2 次,2 例 (6%) 患者接受了 3 次 JAKi。特异性 JAKi 包括 27 例 (79%) 的 ruxolitinib、5 例 (15%) 的莫莫替尼、3 例 (9%) 的 fedratinib、3 例 (9%) 的 pacritinib 和 BMS 911543 JAKi,作为 5 例 (15%) 患者临床试验的一部分。既往治疗还包括 4 例患者的脾放疗 (脾切除术前中位 0.6 年 [0.2-1.2]);4 例患者术前进行脾栓塞术;1 例患者在脾切除术前 10 个月接受了栓塞术。从 MF 诊断到脾切除术的中位间隔为 10.3 年 (1.0-35.4),从开始 JAKi 治疗到脾切除术为 1.8 年 (0.03-12.1)。脾切除术最常见的适应症包括 27 例 (79%) 患者的症状性脾肿大,15 例 (44%) 的输血依赖性贫血,以及 14 例 (41%) 患者准备 ASCT。总体而言,23 例 (68%) 患者在脾切除术前停用了 JAKi,而其余 11 例患者在手术期间/手术后继续使用 JAKi。































 京公网安备 11010802027423号
京公网安备 11010802027423号