当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling Phenanthrenoid Dimerization in Juncus acutus: A DFT-Guided Exploration of Radical-Coupling Reaction Mechanisms
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-12-23 , DOI: 10.1002/ejoc.202401123 Federico Coppola, Paola Cimino, Nadia Rega, Simona Zuppolini, Giovanni Di Fabio, Armando Zarrelli
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-12-23 , DOI: 10.1002/ejoc.202401123 Federico Coppola, Paola Cimino, Nadia Rega, Simona Zuppolini, Giovanni Di Fabio, Armando Zarrelli
|
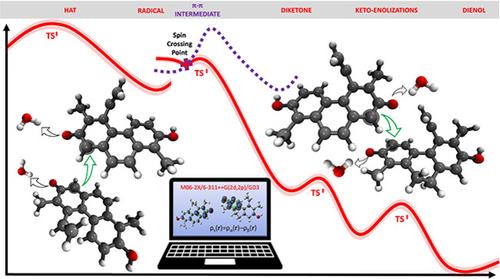
From radical initiation to dimer formation, the radical coupling reaction profile of Juncus acutus phenanthrenoids is mapped through a two-state mechanism, involving a triplet intermediate. This study offers crucial insights into the dimerization process, using computational methods to uncover molecular mechanisms and highlight the role of water in facilitating keto-enol tautomerization, advancing our understanding of biosynthesis in plants.
中文翻译:

揭示 Juncus acutus 中的菲二聚化:DFT 指导的自由基偶联反应机制探索
从自由基起始到二聚体形成,Juncus acutus phenanthrenoids 的自由基偶联反应曲线通过涉及三重态中间体的双态机制进行映射。这项研究为二聚化过程提供了重要的见解,使用计算方法揭示了分子机制,并强调了水在促进酮-烯醇互变异构化中的作用,促进了我们对植物生物合成的理解。
更新日期:2024-12-23
中文翻译:

揭示 Juncus acutus 中的菲二聚化:DFT 指导的自由基偶联反应机制探索
从自由基起始到二聚体形成,Juncus acutus phenanthrenoids 的自由基偶联反应曲线通过涉及三重态中间体的双态机制进行映射。这项研究为二聚化过程提供了重要的见解,使用计算方法揭示了分子机制,并强调了水在促进酮-烯醇互变异构化中的作用,促进了我们对植物生物合成的理解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号