当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Pt Interacts with CeO2 under the Reducing and Oxidizing Environments at Elevated Temperature: The Origin of Improved Thermal Stability of Pt/CeO2 Compared to CeO2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-11-09 00:00:00 , DOI: 10.1021/acs.jpcc.6b08656 Jaeha Lee 1 , YoungSeok Ryou 1 , Xiaojun Chan 2 , Tae Jin Kim 2 , Do Heui Kim 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-11-09 00:00:00 , DOI: 10.1021/acs.jpcc.6b08656 Jaeha Lee 1 , YoungSeok Ryou 1 , Xiaojun Chan 2 , Tae Jin Kim 2 , Do Heui Kim 1
Affiliation
|
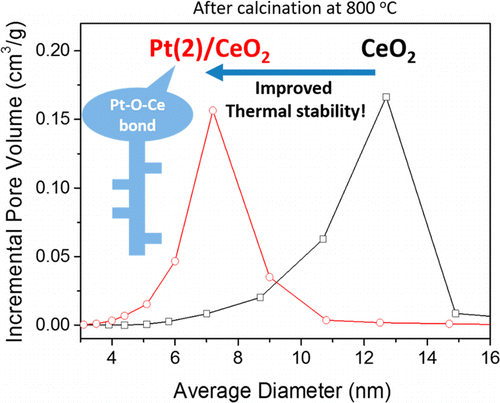
The interaction between Pt and CeO2 under reducing and oxidizing conditions as well as its effect on the thermal stability of Pt/CeO2 were extensively investigated by means of N2 adsorption/desorption, Raman spectroscopy, CO chemisorption, H2 TPR, XRD, and XPS techniques. In situ and ex situ Raman spectroscopy showed that Pt is anchored with the surface oxygen of CeO2 by forming Pt–O–Ce bond during the oxidative treatment of Pt/CeO2. Under the reducing condition, the static CO chemisorption presented that the amount of CO adsorbed on CeO2 is almost equal to that on Pt/CeO2, implying that Pt atom is located on the oxygen vacancy generated on reduced CeO2 surface. Strong Pt–O–Ce bond maintained the textural properties of Pt/CeO2 from oxidative treatment at temperature as high as 800 °C, as evidenced by the XRD patterns and BJH curves of the samples. Selective removal of the surface oxygen of Pt/CeO2 resulted in the decreased thermal stability of Pt/CeO2 due to the loss of Pt–O–Ce bond. Stronger interaction between Pt and CeO2 is observed when the oxidation temperature was increased from 500 to 800 °C, as evidenced by the shift of the surface reduction peak of Pt/CeO2 in H2 TPR to the higher temperature. It is consistent with in situ Raman spectra of Pt/CeO2, which showed that Pt–O–Ce bond became more resistant to the reduction by H2 after the oxidative treatment at 800 °C. Hence, it is concluded that Pt–O–Ce bond plays an important role in improving the thermal stability of Pt/CeO2 upon the oxidative treatment at high temperature. Based on characterization results, the model is proposed to explain the interaction between Pt and CeO2 under the oxidative treatment.
中文翻译:

如何用铈铂交互2下的还原和氧化环境高温:铂热稳定性提高的起源/负责人2相比首席执行官2
通过N 2吸附/解吸,拉曼光谱,CO化学吸附,H 2 TPR,XRD,XRD等研究了还原和氧化条件下Pt与CeO 2之间的相互作用及其对Pt / CeO 2热稳定性的影响。和XPS技术。原位和异位拉曼光谱表明,在Pt / CeO 2的氧化处理过程中,Pt通过形成Pt-O-Ce键与CeO 2的表面氧结合。在还原条件下,静态CO化学吸附表明,CeO 2上的CO吸附量几乎等于Pt / CeO 2上的CO吸附量。,表明Pt原子位于还原的CeO 2表面产生的氧空位上。Xt谱图和样品的BJH曲线证明,强的Pt-O-Ce键在高达800°C的温度下仍能保持Pt / CeO 2的氧化性能。选择性除去的Pt /铈的表面氧的2导致的Pt /铈的热稳定性降低2由于铂-O-Ce的键的损失。当氧化温度从500升高到800°C时,观察到Pt和CeO 2之间的相互作用更强,这可以通过H 2中Pt / CeO 2的表面还原峰的移动来证明。TPR到较高的温度。这与Pt / CeO 2的原位拉曼光谱是一致的,后者表明Pt–O–Ce键在800°C的氧化处理后变得更能抵抗H 2的还原。因此,可以得出结论,在高温下进行氧化处理后,Pt-O-Ce键在改善Pt / CeO 2的热稳定性中起着重要作用。基于表征结果,提出了该模型以解释氧化处理下Pt和CeO 2之间的相互作用。
更新日期:2016-11-09
中文翻译:

如何用铈铂交互2下的还原和氧化环境高温:铂热稳定性提高的起源/负责人2相比首席执行官2
通过N 2吸附/解吸,拉曼光谱,CO化学吸附,H 2 TPR,XRD,XRD等研究了还原和氧化条件下Pt与CeO 2之间的相互作用及其对Pt / CeO 2热稳定性的影响。和XPS技术。原位和异位拉曼光谱表明,在Pt / CeO 2的氧化处理过程中,Pt通过形成Pt-O-Ce键与CeO 2的表面氧结合。在还原条件下,静态CO化学吸附表明,CeO 2上的CO吸附量几乎等于Pt / CeO 2上的CO吸附量。,表明Pt原子位于还原的CeO 2表面产生的氧空位上。Xt谱图和样品的BJH曲线证明,强的Pt-O-Ce键在高达800°C的温度下仍能保持Pt / CeO 2的氧化性能。选择性除去的Pt /铈的表面氧的2导致的Pt /铈的热稳定性降低2由于铂-O-Ce的键的损失。当氧化温度从500升高到800°C时,观察到Pt和CeO 2之间的相互作用更强,这可以通过H 2中Pt / CeO 2的表面还原峰的移动来证明。TPR到较高的温度。这与Pt / CeO 2的原位拉曼光谱是一致的,后者表明Pt–O–Ce键在800°C的氧化处理后变得更能抵抗H 2的还原。因此,可以得出结论,在高温下进行氧化处理后,Pt-O-Ce键在改善Pt / CeO 2的热稳定性中起着重要作用。基于表征结果,提出了该模型以解释氧化处理下Pt和CeO 2之间的相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号