当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Borane-Mediated Highly Secondary Selective Deoxyfluorination of Alcohols
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-12-23 , DOI: 10.1002/anie.202418495
Dominic R Willcox 1 , Nojus Cironis 1 , Laura Winfrey 1 , Sven Kirschner 1 , Gary S Nichol 1 , Stephen P Thomas 1 , Michael J Ingleson 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-12-23 , DOI: 10.1002/anie.202418495
Dominic R Willcox 1 , Nojus Cironis 1 , Laura Winfrey 1 , Sven Kirschner 1 , Gary S Nichol 1 , Stephen P Thomas 1 , Michael J Ingleson 1
Affiliation
|
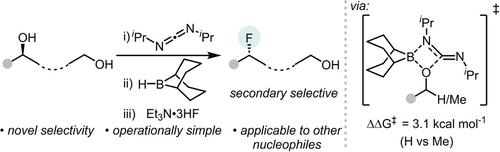
Borane-mediated deoxyfluorination of in situ generated N-H-O-alkyl-isoureas using Et3N ⋅ 3HF represents a novel approach to achieve a highly 2°-alcohol-selective substitution in preference to benzylic, 1° and 3° alcohol deoxyfluorination. The key to the 2° selectivity is the relative stability of the N-BBN-O-alkyl-isoureas towards elimination of the carbodiimide.
中文翻译:

硼烷介导的醇类高度二级选择性脱氧氟化反应
使用 Et3N ⋅ 3HF 对原位生成的 NH-O-烷基异脲进行硼烷介导的脱氧氟化代表了一种优先于苄基、1° 和 3° 醇脱氧氟化实现高度 2°-醇选择性取代的新方法。2° 选择性的关键是 N-BBN-O-烷基异脲对消除碳二亚胺的相对稳定性。
更新日期:2024-12-23
中文翻译:

硼烷介导的醇类高度二级选择性脱氧氟化反应
使用 Et3N ⋅ 3HF 对原位生成的 NH-O-烷基异脲进行硼烷介导的脱氧氟化代表了一种优先于苄基、1° 和 3° 醇脱氧氟化实现高度 2°-醇选择性取代的新方法。2° 选择性的关键是 N-BBN-O-烷基异脲对消除碳二亚胺的相对稳定性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号