当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Potassium Doping on a Two-Dimensional Kagome Organic Framework on Ag(111)
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-12-23 , DOI: 10.1021/acs.jpclett.4c03344 Xingyue Wang, Tianchen Qin, Tian Ma, Sifan You, Jia Wang, Lei Hu, Baiyao Liang, Jun Hu, Dezhou Guo, Minghu Pan, Junfa Zhu, Lifeng Chi
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-12-23 , DOI: 10.1021/acs.jpclett.4c03344 Xingyue Wang, Tianchen Qin, Tian Ma, Sifan You, Jia Wang, Lei Hu, Baiyao Liang, Jun Hu, Dezhou Guo, Minghu Pan, Junfa Zhu, Lifeng Chi
|
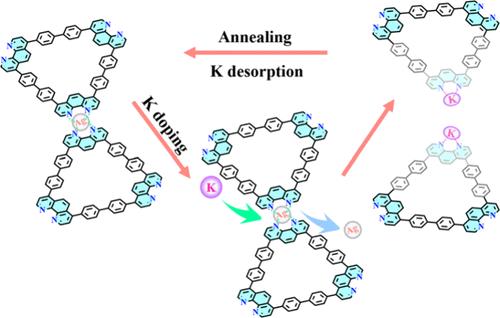
Alkali element doping has significant physical implications for two-dimensional materials, primarily by tuning the electronic structure and carrier concentration. It can enhance interface electronic interactions, providing opportunities for effective charge transfer at metal–organic interfaces. In this work, we investigated the effects of gradually increasing the level of K doping on the lattice structure and electronic properties of an organometallic coordinated Kagome lattice on a Ag(111) surface. With the introduction of K dopants into the 4-fold N–Ag coordinated Kagome lattice, the highly periodic Kagome lattice gradually tends to become discrete. Combining synchrotron radiation photoemission spectroscopy, scanning tunneling microscopy/spectroscopy, and density functional theory calculations, we revealed the mechanism of structural transformation of the lattice, i.e., the change in thermodynamically favored structures caused by competition of electron donors. As an electron donor with a lower ionization energy, K adatoms tend to replace the Ag adatoms and form a more thermodynamically stable N–K coordination structure. Moreover, enhanced charge transfer from K to the Kagome lattice induced a rigid shift of the Fermi level. Our investigation provides new insights for the study of alkali-doped organometallic nanostructures.
中文翻译:

钾掺杂对二维 Kagome 有机框架对 Ag 的影响(111)
碱元素掺杂对二维材料具有重要的物理意义,主要是通过调整电子结构和载流子浓度。它可以增强界面电子相互作用,为金属-有机界面的有效电荷转移提供机会。在这项工作中,我们研究了逐渐增加 K 掺杂水平对 Ag(111) 表面上有机金属配位 Kagome 晶格的晶格结构和电子性质的影响。随着 K 掺杂剂被引入 4 倍 N-Ag 配位的 Kagome 晶格中,高度周期性的 Kagome 晶格逐渐趋于离散。结合同步辐射光电子能谱、扫描隧道显微镜/光谱和密度泛函理论计算,我们揭示了晶格结构转变的机制,即电子供体竞争引起的热力学有利结构的变化。作为具有较低电离能的电子供体,K 吸附原子倾向于取代 Ag 吸附原子并形成热力学上更稳定的 N-K 配位结构。此外,从 K 到 Kagome 晶格的电荷转移增强导致费米能级的刚性偏移。本研究为碱掺杂有机金属纳米结构的研究提供了新的见解。
更新日期:2024-12-23
中文翻译:

钾掺杂对二维 Kagome 有机框架对 Ag 的影响(111)
碱元素掺杂对二维材料具有重要的物理意义,主要是通过调整电子结构和载流子浓度。它可以增强界面电子相互作用,为金属-有机界面的有效电荷转移提供机会。在这项工作中,我们研究了逐渐增加 K 掺杂水平对 Ag(111) 表面上有机金属配位 Kagome 晶格的晶格结构和电子性质的影响。随着 K 掺杂剂被引入 4 倍 N-Ag 配位的 Kagome 晶格中,高度周期性的 Kagome 晶格逐渐趋于离散。结合同步辐射光电子能谱、扫描隧道显微镜/光谱和密度泛函理论计算,我们揭示了晶格结构转变的机制,即电子供体竞争引起的热力学有利结构的变化。作为具有较低电离能的电子供体,K 吸附原子倾向于取代 Ag 吸附原子并形成热力学上更稳定的 N-K 配位结构。此外,从 K 到 Kagome 晶格的电荷转移增强导致费米能级的刚性偏移。本研究为碱掺杂有机金属纳米结构的研究提供了新的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号