当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Sequential Nitro-Michael Addition and Reductive Cyclization Cascade Reaction for Diastereoselective Synthesis of Multifunctionalized 3,3′-pyrrolidinyl-spirooxindoles
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-12-23 , DOI: 10.1002/ejoc.202401121 Chandrakant B. Nichinde, Meema Bhati, Amardipsing S. Girase, Baliram R. Patil, Suryakant S. Chaudhari, Rama Krishna Gamidi, Kavita Joshi, Anil K. Kinage
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-12-23 , DOI: 10.1002/ejoc.202401121 Chandrakant B. Nichinde, Meema Bhati, Amardipsing S. Girase, Baliram R. Patil, Suryakant S. Chaudhari, Rama Krishna Gamidi, Kavita Joshi, Anil K. Kinage
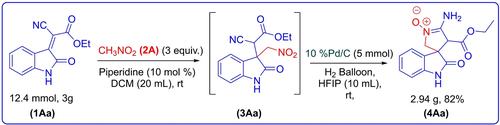
|
An efficient and pot economic synthetic strategy has been established for the diastereoselective construction of multifunctionalized 3,3′-pyrrolidonyl-spirooxindoles. DFT studies clarified the mechanism of regioselective cascade ring closure, highlighting the preferential reactivity of reduced nitro groups with nitriles, while ester groups remained unreactive.
中文翻译:

用于多功能 3,3′-吡咯烷基-螺氧吲哚的非对映选择性合成的连续硝基-迈克尔加成和还原环化级联反应
已经建立了一种高效且经济的合成策略,用于多功能化 3,3′-吡咯己烯基-螺氧吲哚的非对映选择性构建。DFT 研究阐明了区域选择性级联环闭合的机制,突出了还原硝基与腈的优先反应性,而酯基保持无反应性。
更新日期:2024-12-23
中文翻译:

用于多功能 3,3′-吡咯烷基-螺氧吲哚的非对映选择性合成的连续硝基-迈克尔加成和还原环化级联反应
已经建立了一种高效且经济的合成策略,用于多功能化 3,3′-吡咯己烯基-螺氧吲哚的非对映选择性构建。DFT 研究阐明了区域选择性级联环闭合的机制,突出了还原硝基与腈的优先反应性,而酯基保持无反应性。































 京公网安备 11010802027423号
京公网安备 11010802027423号