当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of Unsymmetric Chiral Dialdehydes with Lysine: Regio- and Enantioselective Macrocyclization and Fluorescent Sensing
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-12-23 , DOI: 10.1002/ejoc.202401250 Yifan Mao, Yichen Li, Stephanie Davis, Lin Pu
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-12-23 , DOI: 10.1002/ejoc.202401250 Yifan Mao, Yichen Li, Stephanie Davis, Lin Pu
|
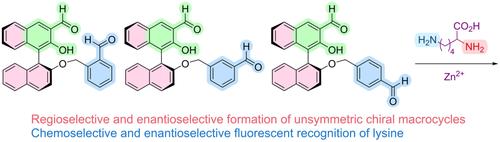
The BINOL-based unsymmetric chiral dialdehydes are discovered to undergo regioselective as well as enantioselective reactions with an unsymmetric chiral diamine, lysine, to generate unsymmetric chiral macrocycles. Addition of Zn2+ can further enhance the selectivity for the macrocycle formation. These compounds are also found to exhibit chemoselective and enantioselective fluorescent recognition of lysine in the presence of Zn2+.
中文翻译:

不对称手性二醛与赖氨酸的反应:区域和对映选择性大环化和荧光传感
发现基于 BINO 的不对称手性二醛与不对称的手性二胺赖氨酸发生区域选择性和对映选择性反应,以产生不对称的手性大环。添加 Zn2+ 可以进一步增强大环形成的选择性。还发现这些化合物在 Zn2+ 存在下表现出对赖氨酸的化学选择性和对映选择性荧光识别。
更新日期:2024-12-23
中文翻译:

不对称手性二醛与赖氨酸的反应:区域和对映选择性大环化和荧光传感
发现基于 BINO 的不对称手性二醛与不对称的手性二胺赖氨酸发生区域选择性和对映选择性反应,以产生不对称的手性大环。添加 Zn2+ 可以进一步增强大环形成的选择性。还发现这些化合物在 Zn2+ 存在下表现出对赖氨酸的化学选择性和对映选择性荧光识别。































 京公网安备 11010802027423号
京公网安备 11010802027423号