当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tunable Arylative Cyclization of 1,6-Enynes Triggered by Rhodium(III)-Catalyzed C–H Activation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-10-22 , DOI: 10.1021/ja5072702 Yuki Fukui 1 , Ping Liu 1 , Qiang Liu 1 , Zhi-Tao He 1 , Nuo-Yi Wu 1 , Ping Tian 1 , Guo-Qiang Lin 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-10-22 , DOI: 10.1021/ja5072702 Yuki Fukui 1 , Ping Liu 1 , Qiang Liu 1 , Zhi-Tao He 1 , Nuo-Yi Wu 1 , Ping Tian 1 , Guo-Qiang Lin 1
Affiliation
|
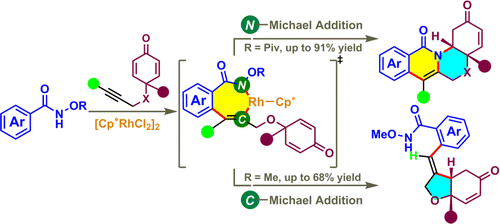
Two tunable arylative cyclizations of cyclohexadienone-containing 1,6-enynes are reported via rhodium(III)-catalyzed C-H activation of O-substituted N-hydroxybenzamides. The use of different O substituents, i.e. O-Piv and O-Me, on the directing group allows the formation of either tetracyclic isoquinolones through an Ⓝ-Michael addition process or hydrobenzofurans through a Ⓒ-Michael addition process. Mechanistic investigations of these two cascade reactions clearly indicated that the C-H bond cleavage process was involved in the turnover-limiting step. Furthermore, the cyclization products could be subjected to various transformations for elaborating the pharmaceutically and synthetically valuable potential. This is the first example of a rhodium(III)-catalyzed arylative cyclization reaction of 1,6-enynes, and the results extend the application realm of Cp*Rh(III)-catalyzed C-H activation cascade reactions.
中文翻译:

由铑 (III) 催化的 C-H 活化触发的 1,6-烯炔的可调芳基环化
通过铑(III)催化的CH活化O-取代的N-羟基苯甲酰胺,报道了含环己二烯酮的1,6-烯炔的两种可调芳基化环化。在导向基团上使用不同的 O 取代基,即 O-Piv 和 O-Me,允许通过Ⓝ-迈克尔加成过程形成四环异喹诺酮或通过Ⓒ-迈克尔加成过程形成氢苯并呋喃。这两个级联反应的机理研究清楚地表明 CH 键裂解过程参与了周转限制步骤。此外,环化产物可以进行各种转化,以发挥药学和合成价值的潜力。这是铑 (III) 催化的 1,6-烯炔芳基化环化反应的第一个例子,
更新日期:2014-10-22
中文翻译:

由铑 (III) 催化的 C-H 活化触发的 1,6-烯炔的可调芳基环化
通过铑(III)催化的CH活化O-取代的N-羟基苯甲酰胺,报道了含环己二烯酮的1,6-烯炔的两种可调芳基化环化。在导向基团上使用不同的 O 取代基,即 O-Piv 和 O-Me,允许通过Ⓝ-迈克尔加成过程形成四环异喹诺酮或通过Ⓒ-迈克尔加成过程形成氢苯并呋喃。这两个级联反应的机理研究清楚地表明 CH 键裂解过程参与了周转限制步骤。此外,环化产物可以进行各种转化,以发挥药学和合成价值的潜力。这是铑 (III) 催化的 1,6-烯炔芳基化环化反应的第一个例子,













































 京公网安备 11010802027423号
京公网安备 11010802027423号