当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glyphosate binding and speciation at the water-goethite interface: A surface complexation model consistent with IR spectroscopy and MO/DFT
Water Research ( IF 11.4 ) Pub Date : 2024-12-21 , DOI: 10.1016/j.watres.2024.123031 Bram Geysels, Tjisse Hiemstra, Jan E. Groenenberg, Rob N.J. Comans
Water Research ( IF 11.4 ) Pub Date : 2024-12-21 , DOI: 10.1016/j.watres.2024.123031 Bram Geysels, Tjisse Hiemstra, Jan E. Groenenberg, Rob N.J. Comans
|
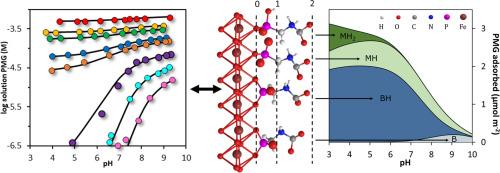
Binding of glyphosate (PMG) to metal (hydr)oxides controls its availability and mobility in natural waters and soils, and these minerals are often suggested for the removal of PMG from wastewaters. However, a solid mechanistic and quantitative description of the adsorption behavior and surface speciation on these surfaces is still lacking, while it is essential for understanding PMG behavior in aquatic and terrestrial systems. This study gives new insights through advanced surface complexation modeling of new and previously published adsorption data, supplemented with MO/DFT calculations of the geometry, thermochemistry and theoretical infrared (IR) spectra of the surface complexes. PMG complexation by goethite (FeOOH) was measured over a wide range of pH (∼4–10), solution concentration (∼10–7 –10-3 M), and surface loading (∼0.3–3.0 μmol m-2 ). Mechanistical modeling using the charge distribution approach revealed the formation of both monodentate and bidentate PMG complexes, each in two protonation states. PMG adsorption is dominated (>60 %) by the formation of a bidentate complex having a protonated amino group that deprotonates at high pH and low loading, aligning with previously published ATR-FTIR analyses. Monodentate complexes are less abundant and maintain a protonated amino group over the entire pH range. In addition, the phosphonate group becomes protonated at low pH and high loading. DFT calculations support the role of protons in the surface speciation. The obtained model was able to predict the solution concentration of PMG and its strong pH dependency over the full range in our experiments. Our study provides a new mechanistic and quantitative understanding of PMG binding to goethite, which enables improved predictions of the fate and transport of PMG in and towards natural waters, and provides a framework for optimizing the removal efficiency of PMG with metal (hydr)oxides.
中文翻译:

水-针铁矿界面处的草甘膦结合和形态形成:与红外光谱和 MO/DFT 一致的表面络合模型
草甘膦 (PMG) 与金属(氢)氧化物的结合控制了其在自然水和土壤中的可用性和流动性,这些矿物质通常被建议用于从废水中去除 PMG。然而,仍然缺乏对这些表面上的吸附行为和表面形态的可靠机械和定量描述,而这对于理解 PMG 在水生和陆地系统中的行为至关重要。本研究通过对新的和以前发表的吸附数据进行高级表面络合建模,并辅以表面络合物的几何形状、热化学和理论红外 (IR) 光谱的 MO/DFT 计算,提供了新的见解。针铁矿 (FeOOH) 在较宽的 pH 值 (∼4–10)、溶液浓度 (∼10–7–10-3M) 和表面负载 (∼0.3–3.0 μmol m-2) 范围内测量针铁矿 (FeOOH) 的 PMG 络合。使用电荷分布方法的机理建模揭示了单齿和双齿 PMG 复合物的形成,每种复合物都处于两种质子化状态。PMG 吸附主要 (>60 %) 由形成双齿络合物为主,该络合物具有质子化氨基,在高 pH 值和低负载下去质子化,与以前发表的 ATR-FTIR 分析一致。单齿复合物的丰度较低,并且在整个 pH 范围内保持质子化氨基。此外,膦酸盐基团在低 pH 值和高负载量下会质子化。DFT 计算支持质子在表面形态形成中的作用。在我们的实验中,获得的模型能够预测 PMG 的溶液浓度及其在整个范围内的强烈 pH 依赖性。 我们的研究为PMG与针铁矿的结合提供了新的机理和定量理解,从而可以更好地预测PMG在自然水域中的命运和运输,并为优化PMG与金属(氢)氧化物的去除效率提供了一个框架。
更新日期:2024-12-21
中文翻译:

水-针铁矿界面处的草甘膦结合和形态形成:与红外光谱和 MO/DFT 一致的表面络合模型
草甘膦 (PMG) 与金属(氢)氧化物的结合控制了其在自然水和土壤中的可用性和流动性,这些矿物质通常被建议用于从废水中去除 PMG。然而,仍然缺乏对这些表面上的吸附行为和表面形态的可靠机械和定量描述,而这对于理解 PMG 在水生和陆地系统中的行为至关重要。本研究通过对新的和以前发表的吸附数据进行高级表面络合建模,并辅以表面络合物的几何形状、热化学和理论红外 (IR) 光谱的 MO/DFT 计算,提供了新的见解。针铁矿 (FeOOH) 在较宽的 pH 值 (∼4–10)、溶液浓度 (∼10–7–10-3M) 和表面负载 (∼0.3–3.0 μmol m-2) 范围内测量针铁矿 (FeOOH) 的 PMG 络合。使用电荷分布方法的机理建模揭示了单齿和双齿 PMG 复合物的形成,每种复合物都处于两种质子化状态。PMG 吸附主要 (>60 %) 由形成双齿络合物为主,该络合物具有质子化氨基,在高 pH 值和低负载下去质子化,与以前发表的 ATR-FTIR 分析一致。单齿复合物的丰度较低,并且在整个 pH 范围内保持质子化氨基。此外,膦酸盐基团在低 pH 值和高负载量下会质子化。DFT 计算支持质子在表面形态形成中的作用。在我们的实验中,获得的模型能够预测 PMG 的溶液浓度及其在整个范围内的强烈 pH 依赖性。 我们的研究为PMG与针铁矿的结合提供了新的机理和定量理解,从而可以更好地预测PMG在自然水域中的命运和运输,并为优化PMG与金属(氢)氧化物的去除效率提供了一个框架。

































 京公网安备 11010802027423号
京公网安备 11010802027423号