当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Anions and Water Content on [Li–Al] Layered Double-Hydroxide Stability
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.jpcc.4c06793 Christopher Hoban, Natalie Parkinson, K. Jayanthi, M. Parans Paranthaman, Alexandra Navrotsky, Brian F. Woodfield
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.jpcc.4c06793 Christopher Hoban, Natalie Parkinson, K. Jayanthi, M. Parans Paranthaman, Alexandra Navrotsky, Brian F. Woodfield
|
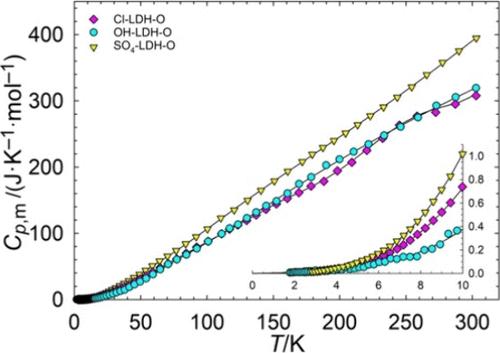
[Li–Al] layered double hydroxides (LDHs) are compounds with potential as sorbents for lithium extraction from brine solutions. In this work, heat capacities were measured from approximately 2.5 to 300 K for six [Li–Al] LDHs with differing anions (Cl–, OH–, and SO42–) and water content (denoted A for air-dried and O for oven-dried). These measurements were used to calculate the standard entropy at 298.15 K, and the results were combined with previously performed enthalpy measurements to calculate Gibbs energies of formation from the binary compounds. The calculated order of stability based on Gibbs energies of formation was Cl-LDH-O > OH-LDH-O > Cl-LDH-A > SO4-LDH-O > SO4-LDH-A > OH-LDH-A. Results support previous findings that higher water content generally raises the Gibbs energy of the LDH.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号