当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generation and Application of All Possible Conformations of Cyclic Tryptophan within and beyond Post-translational Modification
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-20 , DOI: 10.1021/acs.joc.4c02532 Akitomo Kasahara, Ryo Yamada, Tadashi Hyodo, Kentaro Yamaguchi, Yuko Otani, Shimpei Sumimoto, Masahiro Okada, Tomohiko Ohwada
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-20 , DOI: 10.1021/acs.joc.4c02532 Akitomo Kasahara, Ryo Yamada, Tadashi Hyodo, Kentaro Yamaguchi, Yuko Otani, Shimpei Sumimoto, Masahiro Okada, Tomohiko Ohwada
|
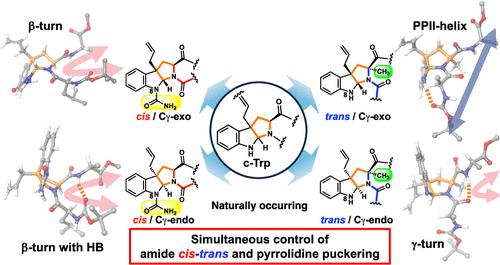
Isoprenylation of the indole C3-position of tryptophan accompanied by cyclization (c-Trp) is one of the most attractive post-translational modifications because of C–C bond formation and drastic conformational alteration. As the modification generates two stereoisomers of the 6/5/5-fused ring system and consequently, a mixture of four possible conformations as considered in proline, it is expected to influence the biological activity in Bacillus quorum sensing pheromone ComX containing the c-Trp residue. In this study, the simultaneous control of the amide cis–trans equilibrium and pyrrolidine ring puckering was achieved by utilizing an N-carbamoylated and α-methylated 6/5/5-fused ring system. Furthermore, the conformationally defined tripeptides containing the c-Trp residue were utilized to examine the relationship between the biological activity and the conformation of the ComX pheromone. Several mimics showed high bioactivity, and more biologically active ComX mimics were created to reinforce the CH-π interaction of the c-Trp and the adjacent aromatic residue.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号