当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competing Photocleavage on Boron and at the meso-Position in BODIPY Photocages
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.joc.4c02226 Ivan Ljubić, Igor Sviben, Vedran Brusar, Katarina Zlatić, Silvije Vdović, Nikola Basarić
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.joc.4c02226 Ivan Ljubić, Igor Sviben, Vedran Brusar, Katarina Zlatić, Silvije Vdović, Nikola Basarić
|
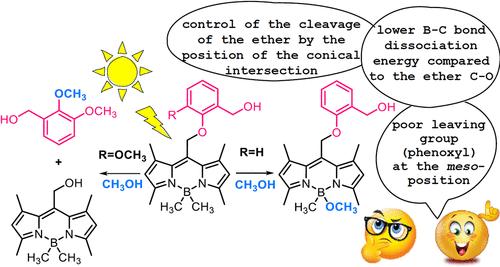
BODIPY photocages (photocleavable protective groups) have stirred interest because they can release biologically active cargo upon visible light excitation. We conducted combined theoretical and experimental investigations on selected BODIPY photocages to elucidate the mechanism of the competing photocleavage at the boron and meso-position. Based on the computations, the former reaction involves elongation of the B–C bond, yielding a tight borenium cation and methyl anion. These ions are intercepted by CH3OH, enabling an efficient proton-coupled electron transfer (PCET) to produce the methane and isolated ether photoproducts. Singlet and triplet excited-state lifetimes were measured in CH3OH and CD3OD to probe the kinetic isotope effects (KIEs). The resulting KIEs are small, implying that the kinetic bottleneck is due to the C–B bond scission rather than the subsequent PCET. The introduction of a methoxy group in the meso-phenoxy substituent redirects the photosubstitution toward the meso-position. The corresponding regiochemistry was explained computationally. On elongating the C–O bonds in the S1 state, it is found that the unproductive conical intersection is encountered much earlier for the alkyl–O bond than for the phenyl–O bond. The current findings are valuable for the rational design of new BODIPY photocages with tailored biological applications.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号