当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Protecting Groups and Activating Conditions on 3-Deoxy-d-glycero-d-galacto-2-nonulosonic Acid (Kdn) Glycosylation: Stereoselective Synthesis of α- and β-Kdn Glycosides
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-20 , DOI: 10.1021/acs.orglett.4c04325 Jin-Song Yang, Yu-Xiong Ruan, Hong-Yang Wang, Ling Li, Yan-Li Zhao, Yong Qin
Organic Letters ( IF 4.9 ) Pub Date : 2024-12-20 , DOI: 10.1021/acs.orglett.4c04325 Jin-Song Yang, Yu-Xiong Ruan, Hong-Yang Wang, Ling Li, Yan-Li Zhao, Yong Qin
|
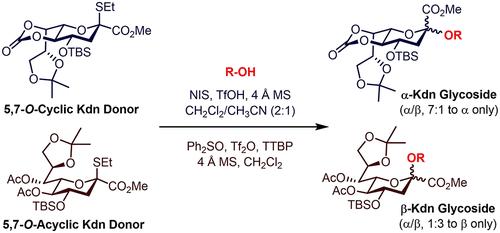
Kdn is a common member of the sialic acid family. Carbohydrates containing Kdn residues are widely distributed in nature and embody important biological information. However, the methods for synthesizing Kdn glycosides are limited, which restricts their biological study. In this paper, we developed efficient α- and β-stereoselective Kdn glycosylation methods by employing differentially protected Kdn thioglycoside donors under their respective activating protocols. The 5,7-O-carbonate fused Kdn thioglycoside 1a could be promoted with NIS/TfOH (cat.) in CH2Cl2/CH3CN (2:1) to afford Kdn glycosides with excellent α-selectivity in high yields. Meanwhile, based on the Ph2SO/Tf2O preactivation strategy, the nonfused Kdn thioglycoside 1b behaved as a high-yielding and β-selective donor to couple with various carbohydrate alcohols, leading to formation of β-Kdn glycosides. The synthetic utility of these newly developed glycosyl donors has been demonstrated by the stereoselective and straightforward assembly of two natural Kdn-containing oligosaccharides.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号