Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cavitation, Hydrophilicity, and Sorption Hysteresis in C–S–H Pores: Coupled Effects of Relative Humidity and Temperature
Langmuir ( IF 3.7 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.langmuir.4c03223 Fatima Masara, Farid Benboudjema, Tulio Honorio
Langmuir ( IF 3.7 ) Pub Date : 2024-12-19 , DOI: 10.1021/acs.langmuir.4c03223 Fatima Masara, Farid Benboudjema, Tulio Honorio
|
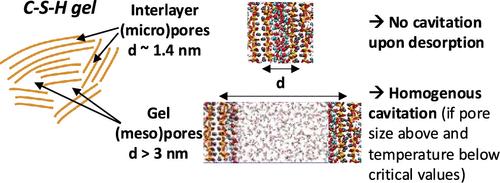
Sorption processes are critical for the drying and durability of cement-based materials, directly affecting their thermal properties. Temperature can substantially influence these processes. This work uses molecular simulations to study sorption in C–S–H pores under varying temperatures and relative humidity, considering pore sizes from the gel to the interlayer scale (between 11.6 and 106 Å). We quantify the temperature and pore-size dependence of water cavitation and sorption hysteresis in the C–S–H pores. The critical pore sizes for the disappearance of hysteresis and the reversibility of capillary condensation are identified, with the former being directly associated with cavitation. We show that cavitation occurs only in gel (meso)pores when they are above the critical pore size and below the critical temperature for cavitation. Interlayer pores, a major class of micropores in C–S–H, are not subjected to cavitation. Cavitation in C–S–H pores is homogeneous, occurring in the bulk-like zone of mesopores. The hydrophilicity of the C–S–H surface increases with the temperature, making heterogeneous cavitation less likely to occur. The results above were obtained consistently with three different force field parametrizations, building confidence in their relevance to describe C–S–H interfacial behavior. Finally, we demonstrate that macroscopic considerations for pore emptying and filling, such as the Kelvin-Cohan and equilibrium Derjaguin-Broekhoff-de Boer equations, are not valid or inaccurate when desorption occurs through cavitation in C–S–H. These results are relevant to understanding the sorption processes in other nanolayered adsorbing materials.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号