Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antigen presentation by MHC-II is shaped by competitive and cooperative allosteric mechanisms of peptide exchange
Structure ( IF 4.4 ) Pub Date : 2024-12-20 , DOI: 10.1016/j.str.2024.11.014 Matthias Günther, Jana Sticht, Christian Freund, Thomas Höfer
Structure ( IF 4.4 ) Pub Date : 2024-12-20 , DOI: 10.1016/j.str.2024.11.014 Matthias Günther, Jana Sticht, Christian Freund, Thomas Höfer
|
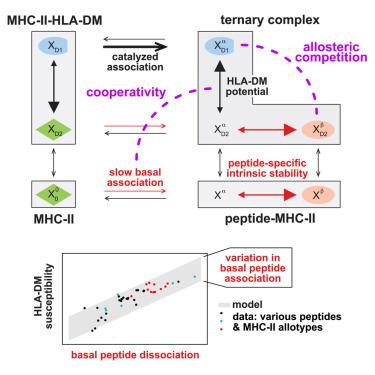
Major histocompatibility complex class II (MHC-II) presents antigens to T helper cells. The spectrum of presented peptides is regulated by the exchange catalyst human leukocyte antigen DM (HLA-DM), which dissociates peptide-MHC-II complexes in the endosome. How susceptible a peptide is to HLA-DM is mechanistically not understood. Here, we present a data-driven mathematical model for the conformational landscape of MHC-II that explains the wide range of measured HLA-DM susceptibilities and predicts why some peptides are largely HLA-DM-resistant. We find that the conformational plasticity of MHC-II mediates both allosteric competition and cooperation between peptide and HLA-DM. Competition causes HLA-DM susceptibility to be proportional to the intrinsic peptide off-rate. Remarkably, diverse MHC-II allotypes with conserved HLA-DM interactions show a universal linear susceptibility function. However, HLA-DM-resistant peptides deviate from this susceptibility function; we predict resistance to be caused by fast peptide association with MHC-II. Thus, our study provides quantitative insight into peptide and MHC-II allotype parameters that shape class-II antigen presentation.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号