当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bicarbonate ions promote rapid degradation of pollutants in Co(II)Fe(II)/peroxyacetic acid systems
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-20 , DOI: 10.1016/j.jhazmat.2024.136918 Yuqiong Wang, Zonghui Zhang, Lele Zhao, Chong Ma, Qi Hu, Xiaohong Hou
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-20 , DOI: 10.1016/j.jhazmat.2024.136918 Yuqiong Wang, Zonghui Zhang, Lele Zhao, Chong Ma, Qi Hu, Xiaohong Hou
|
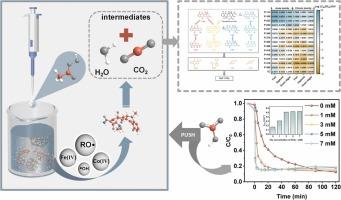
Peroxyacetic acid (PAA), as an oxidizing agent, has gained significant attention in the field of advanced oxidation because of its low toxicity and high degradation capacity. In this study, cobalt-iron-based Prussian blue analogs (Co-PBAs) were utilized for the first time to activate PAA for tetracycline degradation. In the Co-PBAs/PAA system, organic radicals (RO•) and high-valent metal oxides are mainly produced. TC is efficiently removed in a wide pH range (5−9) and a variety of interferences (Cl- , SO4 2- , bicarbonate ions (HCO3 - ), humic acid, and the actual water bodies) in water bodies due to the specificity of RO•. Interestingly, the catalytic rate of the Co-PBAs/PAA system was significantly accelerated in the presence of HCO3 - (kobs increasing from 0.171 min−1 to 0.534 min−1 ). This enhancement is attributed to the reaction between HCO3 - and PAA, and carbonate radicals (•CO3 - ) and acetyl peroxyl radicals (CH3 C(O)OO•) are generated and then react with the phenolic hydroxyl group of TC. In this study, the mechanism of PAA activation by Co-PBAs was revealed, and PAA-based advanced oxidation process enhanced by HCO3 - was provided for the removal of pollutants from wastewater.
中文翻译:

碳酸氢根离子促进 Co(II)Fe(II)/过氧乙酸体系中污染物的快速降解
过氧乙酸 (PAA) 作为一种氧化剂,因其低毒性和高降解能力而在高级氧化领域受到了极大的关注。在这项研究中,首次使用钴铁基普鲁士蓝类似物 (Co-PBA) 来激活 PAA 进行四环素降解。在 Co-PBAs/PAA 系统中,主要产生有机自由基 (RO•) 和高价金属氧化物。由于 RO•的特异性,TC 在较宽的 pH 范围 (5-9) 和水体中的各种干扰物(Cl-、SO42-、碳酸氢根离子 (HCO3-)、腐殖酸和实际水体)中被有效去除。有趣的是,在 HCO3- 存在下,Co-PBAs/PAA 系统的催化速率显着加快 (kobs 从 0.171 min-1 增加到 0.534 min-1)。这种增强归因于 HCO3- 和 PAA 之间的反应,生成碳酸盐自由基 (•CO3-) 和乙酰过氧自由基 (CH3C(O)OO•),然后与 TC 的酚羟基反应。在本研究中,揭示了 Co-PBAs 激活 PAA 的机制,并提供了由 HCO3- 增强的基于 PAA 的高级氧化过程以去除废水中的污染物。
更新日期:2024-12-20
中文翻译:

碳酸氢根离子促进 Co(II)Fe(II)/过氧乙酸体系中污染物的快速降解
过氧乙酸 (PAA) 作为一种氧化剂,因其低毒性和高降解能力而在高级氧化领域受到了极大的关注。在这项研究中,首次使用钴铁基普鲁士蓝类似物 (Co-PBA) 来激活 PAA 进行四环素降解。在 Co-PBAs/PAA 系统中,主要产生有机自由基 (RO•) 和高价金属氧化物。由于 RO•的特异性,TC 在较宽的 pH 范围 (5-9) 和水体中的各种干扰物(Cl-、SO42-、碳酸氢根离子 (HCO3-)、腐殖酸和实际水体)中被有效去除。有趣的是,在 HCO3- 存在下,Co-PBAs/PAA 系统的催化速率显着加快 (kobs 从 0.171 min-1 增加到 0.534 min-1)。这种增强归因于 HCO3- 和 PAA 之间的反应,生成碳酸盐自由基 (•CO3-) 和乙酰过氧自由基 (CH3C(O)OO•),然后与 TC 的酚羟基反应。在本研究中,揭示了 Co-PBAs 激活 PAA 的机制,并提供了由 HCO3- 增强的基于 PAA 的高级氧化过程以去除废水中的污染物。































 京公网安备 11010802027423号
京公网安备 11010802027423号