当前位置:
X-MOL 学术
›
Sens. Actuators B Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-tandem enzymatic modules-encapsulated metal-organic framework cubes with mutual-validated operation for dual-modal competitive immunoassay of antibiotics
Sensors and Actuators B: Chemical ( IF 8.0 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.snb.2024.137160 Yi Yang, Licheng Yu, Liang He, Pengli Bai, Xiwen He, Langxing Chen, Yukui Zhang
Sensors and Actuators B: Chemical ( IF 8.0 ) Pub Date : 2024-12-19 , DOI: 10.1016/j.snb.2024.137160 Yi Yang, Licheng Yu, Liang He, Pengli Bai, Xiwen He, Langxing Chen, Yukui Zhang
|
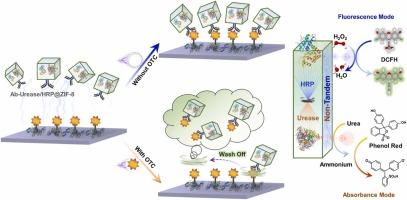
Adaptive fusion of nanoscale metal-organic frameworks (nMOFs) and enzyme-based immunoassay is an insightful combination for inspired establishment of antibiotic assay, typically in a competitive format, in which inherent enzymes in nMOFs can serve as generators for amplifying catalytic signals. Albeit important, there is currently a lack of explorations of enzymatic signal outputs with multiple modalities in typical competitive immunoassays, which may face the false signals and lack cross-validation. Herein, non-tandem bi-enzymatic modules such as urease and horseradish peroxidase were co-encapsulated into nMOF cubes and functionalized with antibodies into entities for dual-modal competitive immunoassay of oxytetracycline (OTC), which can convert the bi-enzymatic catalytic signals into fluorogenic and chromogenic reflection and can gain the mutual validation merits. The fluorescence mode can achieve an OTC detection range from 5 ng/mL to 2000 ng/mL, with a detection limit (LOD) of 2.14 ng/mL; complementally, the absorbance mode can achieve a relatively narrow detection range from 1 ng/mL to 200 ng/mL, but a lower LOD of 0.32 ng/mL. The dual-mode sensing parameters of the competitive immunoassay in responding to OTC analysis differed in terms of the linear range and detection limit, which can be integrated to offer reciprocal authentication and expand the detection diversity. Overall, our proposed method may provide a new idea in dual-modal competitive immunoassay of antibiotics, signifying its promising expansion for rational substitution of enzymatic modules and its potential performance in wide applications.
中文翻译:

非串联酶模块封装的金属有机框架立方体,具有相互验证的操作,用于抗生素的双模式竞争性免疫测定
纳米级金属有机框架 (nMOF) 和基于酶的免疫测定的适应性融合是启发性建立抗生素测定的有见地的组合,通常以竞争形式,其中 nMOF 中的固有酶可以作为放大催化信号的发生器。尽管很重要,但目前在典型的竞争性免疫测定中缺乏对具有多种模式的酶信号输出的探索,这可能面临错误信号并且缺乏交叉验证。在此,将脲酶和辣根过氧化物酶等非串联双酶模块共封装到 nMOF 立方体中,并与抗体功能化成用于土霉素 (OTC) 双模式竞争免疫测定的实体,可以将双酶催化信号转化为荧光和显色反射,并获得相互验证的优点。荧光模式可实现 5 ng/mL 至 2000 ng/mL 的 OTC 检测范围,检测限 (LOD) 为 2.14 ng/mL;互补地,吸光度模式可以实现 1 ng/mL 至 200 ng/mL 的相对较窄的检测范围,但 LOD 较低,为 0.32 ng/mL。竞争性免疫测定响应 OTC 分析的双模式传感参数在线性范围和检测限方面有所不同,可以整合以提供互惠认证并扩大检测多样性。总的来说,我们提出的方法可能为抗生素的双模态竞争免疫分析提供新的思路,标志着其对酶模块合理替代的有希望的扩展及其在广泛应用中的潜在性能。
更新日期:2024-12-21
中文翻译:

非串联酶模块封装的金属有机框架立方体,具有相互验证的操作,用于抗生素的双模式竞争性免疫测定
纳米级金属有机框架 (nMOF) 和基于酶的免疫测定的适应性融合是启发性建立抗生素测定的有见地的组合,通常以竞争形式,其中 nMOF 中的固有酶可以作为放大催化信号的发生器。尽管很重要,但目前在典型的竞争性免疫测定中缺乏对具有多种模式的酶信号输出的探索,这可能面临错误信号并且缺乏交叉验证。在此,将脲酶和辣根过氧化物酶等非串联双酶模块共封装到 nMOF 立方体中,并与抗体功能化成用于土霉素 (OTC) 双模式竞争免疫测定的实体,可以将双酶催化信号转化为荧光和显色反射,并获得相互验证的优点。荧光模式可实现 5 ng/mL 至 2000 ng/mL 的 OTC 检测范围,检测限 (LOD) 为 2.14 ng/mL;互补地,吸光度模式可以实现 1 ng/mL 至 200 ng/mL 的相对较窄的检测范围,但 LOD 较低,为 0.32 ng/mL。竞争性免疫测定响应 OTC 分析的双模式传感参数在线性范围和检测限方面有所不同,可以整合以提供互惠认证并扩大检测多样性。总的来说,我们提出的方法可能为抗生素的双模态竞争免疫分析提供新的思路,标志着其对酶模块合理替代的有希望的扩展及其在广泛应用中的潜在性能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号