当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Synergistic Hybrid of Sr3B2O6‐SryTi0.6Fe0.4O3‐δ (y < 1) as a Cathode for High‐Performance Electrochemical Ammonia Synthesis via Protonic Ceramic Electrolysis Cells
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-12-20 , DOI: 10.1002/adfm.202418404 Kaihui Wang, Wenyan Zan, Yawei Li, Si‐Dian Li, Zongping Shao, Huili Chen
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-12-20 , DOI: 10.1002/adfm.202418404 Kaihui Wang, Wenyan Zan, Yawei Li, Si‐Dian Li, Zongping Shao, Huili Chen
|
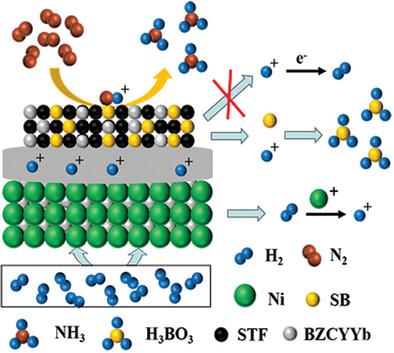
The electrochemical nitrogen reduction reaction (e‐NRR) presents a promising approach for environmentally friendly ammonia synthesis. However, the efficiency of ammonia synthesis can be hindered by competitive hydrogen evolution reactions (HER). Therefore, the development of a catalyst capable of suppressing HER is essential. In this study, a synergistic hybrid catalyst Sr(Ti0.6 Fe0.4 )0.8 B0.2 O3‐δ [S(TF)B0.2 ], composed of Sr3 B2 O6 (SB) and Sry Ti0.6 Fe0.4 O3‐δ (Sy TF, y<1) is synthesized and used as an electrocatalyst for electrochemical ammonia synthesis via protonic ceramic electrolysis cells, in which SB is utilized as a proton acceptor, thereby inhibiting HER and promoting proton‐coupled electron transfer (PCET) for the ammonia synthesis. The formation of SB results in a deficiency of A‐site cations in Sy TF, leading to an increased number of oxygen vacancies in S(TF)B0.2 . DFT calculation indicates that oxygen vacancies facilitate ammonia generation and desorption, adhering to the enzymatic pathway for NH3 synthesis. Additionally, the grain boundary (GB) between Sy TF and SB introduces further defects, which contribute to the enhancement of the eNRR. Research indicates that utilizing S(TF)B0.2 as a catalyst enhances both the ammonia synthesis rate and Faradaic efficiency. This study presents a straightforward and efficient approach for the fabrication of eNRR catalysts.
更新日期:2024-12-20






























 京公网安备 11010802027423号
京公网安备 11010802027423号